8.1 Energy sources
Essential Idea:
The constant need for new energy sources implies decisions that may have a serious effect on the environment. The finite quantity of fossil fuels and their
implication in global warming has led to the development of alternative sources of energy. This continues to be an area of rapidly changing technological innovation.
Understandings:
- Specific energy and energy density of fuel sources
- Sankey diagrams
- Primary energy sources
- Electricity as a secondary and versatile form of energy
- Renewable and non-renewable energy sources
Applications and Skills:
- Solving specific energy and energy density problems
- Sketching and interpreting Sankey diagrams
- Describing the basic features of fossil fuel power stations, nuclear power stations, wind generators, pumped storage hydroelectric systems and solar power cells
- Solving problems relevant to energy transformations in the context of these generating systems
- Discussing safety issues and risks associated with the production of nuclear power
- Describing the differences between photovoltaic cells and solar heating panels
Data booklet reference:
- \(Power=\frac{energy}{time}\)
- \(Power=\frac{1}{2}A\rho v^3\)
Big Ideas
• Most energy sources can be traced back the sun, our ultimate primary source
• Energy sources must be compared based on many factors including energy density, cost, availability, politics, safety, and environmental impact
• No energy source can be converted to electricity with 100% efficiency
• All energy sources have advantages and drawbacks and it important to understand the complete picture
• Every object with a temperature above 0 K emits thermal radiation
• Radiation intensity is related to separation distance by the inverse square law (similar to force fields)
• The Earth’s climate relies on a delicate thermal energy balance where total energy in equals total energy out
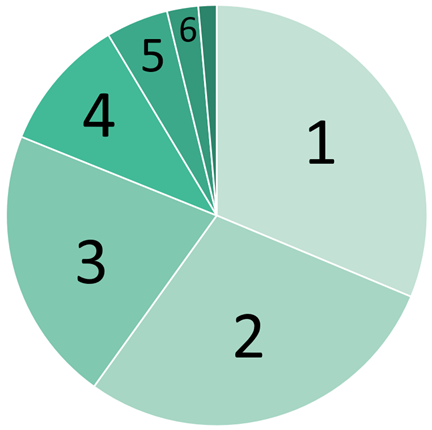
Global Energy Usage
Rank | Energy Source | % |
1 | Oil | 32% |
2 | Coal | 28% |
3 | Natural Gas | 22% |
4 | Biomass | 10% |
5 | Nuclear | 5% |
6 | Hydropower | 2.5% |
Efficiency
Sankey Diagram Rules: Width of the arrow proportional to the amount of energy | 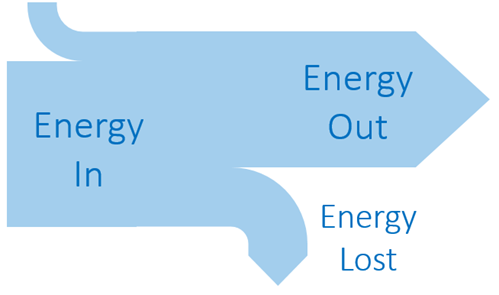 |
Energy Density
Definition | Units | |
Specific Energy | Energy transferred per unit mass | J kg-1 |
Energy Density | Energy transferred per unit volume | J m-3 |
Primary and Secondary Sources
Primary Energy Sources | Secondary Energy Sources |
Energy sources found in the natural environment (fossil fuels, solar, wind, nuclear, hydro, etc.) | Useful transformations of the primary sources (electricity, pumped storage for hydro, etc.) |
Fossil Fuels
Number of years left in global reserves | |
Coal | ~100-150 years |
Oil | ~50 years |
Natural Gas | ~50 years |
Describe the process of Fracking: |
1. Drill hole into shale rock 2. Inject fracking fluid at high pressure to create cracks 3. Extract newly released natural gas 4. Seal fracking fluid in the hole |
Nuclear Power
| % of U-235 |
Uranium Ore | 0.7% |
Fuel-Grade | 3.5% |
Weapons-Grade | 90% |
Why is the concentration of U-235 important? Only U-235 can undergo a fission chain reaction |
What is done with the nuclear waste? Stored on-site in spent fuel pools and/or concrete dry cask storage |
Moderator | Control Rods |
Slows down neutrons to be absorbed by U-235 Made from Water or Graphite (carbon) | Absorbs neutrons to limit number of chain reactions Made from Boron |
Renewable Energy
Variable Symbol | Unit | |
Power | P | W |
Cross-Sectional Area | A | m2 |
Air Density | ρ | kg m-3 |
Air Speed | v | m s-1 |
Data Booklet Equations: |
Photovoltaic Cells | Solar Concentrator | Solar Heating Panel |
Converts solar energy directly into electricity. Useful in solar panels on top of building or solar farms connected to the energy grid | Mirrors focus sunlight onto a central tower. The high thermal energy is converted to steam and runs turbines to produce electricity | Sun’s radiation is absorbed by black pipes that transfer thermal energy to the water flowing through them. Replaces hot water heater. |
| Biomass | Coal | Geothermal | Hydropower | Natural Gas | Nuclear | Petroleum | Solar | Wind |
Renewable | ✓ |
| ✓ | ✓ |
|
|
| ✓ | ✓ |
Produces CO2 | ✓ | ✓ |
|
| ✓ |
| ✓ |
|
|
Thermal Energy Transfer
Conduction | Convection | Radiation |
Energy is transferred through molecular collisions | Energy circulates through the expansion and rising of hot fluids | Energy is transferred through electromagnetic radiation. Can travel through a vacuum |
| Emissivity |
| Black Body Radiation | 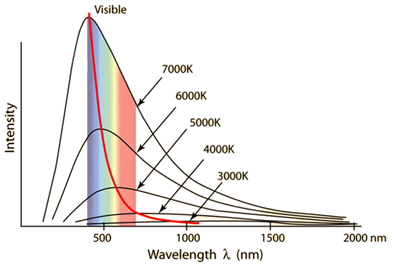 |
Sun | ~1 |
| An idealized object that absorbs all the electromagnetic radiation the falls on it | |
Earth | ~0.6 |
| ||
Black-Body | 1 |
|
Power Emissivity | Variable Symbol | Unit |
Power | P | W |
Emissivity | e | — |
Surface Area | A | m2 |
Temperature | T | K |
Max Wavelength | λmax | m |
Data Booklet Equations: |
Solar Radiation and Climate Change
Intensity | Variable Symbol | Unit |
| Data Booklet Equations: |
Intensity | I | W m-2 |
| |
Power | P | W |
| |
Area | A | m2 |
|
Greenhouse Gases |
| Positive Feedback Loop | Negative Feedback Loop |
Water Vapor (H2O) |
| Melting ice (decreases albedo) | Cloud formation (increases albedo) |
Carbon Dioxide (CO2) |
| Melting permafrost (releases methane) | Increased photosynthesis (uses CO2) |
Methane (CH4) |
| Rising ocean temp releases methane | Climate Change leads to renewables |
Power Delivered by Wind Generator:
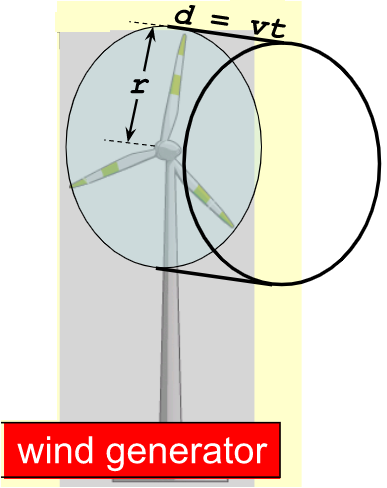
Assume a rotor blade radius of r. ∙
The volume of air that moves through the blades in a time t is given by
V = Ad = Avt, where v is the speed of the air and A = πr2.
The mass m is thus m = ρV = ρAvt.
EK = (1/2)mv2 = (1/2)ρAvtv2 = (1/2)ρAv3t.
Power is \(\frac{E_K}{t}\) so that
\(\frac{E_K}{t}=\frac{1}{2} A\rho v^3\)
Where \(A = \pi r^2\)
Sankey diagrams
Energy degradation in systems can be shown with an energy flow diagram called a Sankey diagram.
Sankey diagrams show the efficiency of each energy conversion.
Suppose the actual energy values are as shown:
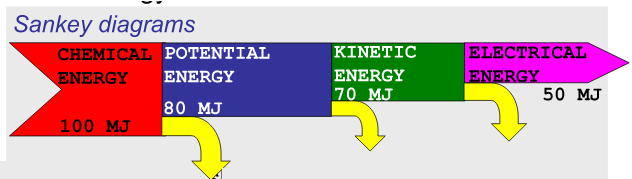
The efficiency of a conversion is given by
\(Efficiency =\frac{output}{Input}\)
For example, the efficiency of the first energy conversion (chemical to potential) is
efficiency = 80 MJ / 100 MJ = 0.80 or 80%.
