- IB Style Question Banks with Solution
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
2.8 Cell Respiration
Essential Idea:
Cell respiration supplies energy for the functions of life
Understandings:
- Cell respiration is the controlled release of energy from organic compounds to produce ATP
- ATP from cell respiration is immediately available as a source of energy in the cell
- Anaerobic cell respiration gives a small yield of ATP from glucose
- Aerobic cell respiration requires oxygen and gives a large yield of ATP from glucose
Applications:
- Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking
- Lactate production in humans when anaerobic respiration is used to maximise the power of muscle contractions
Skills:
- Analysis of results from experiments involving measurement of respiration rates in germinating seeds or invertebrates using a respirometer
- Define “cell respiration.”
- State the reaction for cellular respiration.
- State the types of organic compounds used in cellular respiration by animals and plants.
- State three example uses of cellular energy.
- Outline energy transfer in the formation and use of ATP.
- State three reasons why cellular respiration must be continuously performed by all cells.
- Define “anaerobic respiration”
- List three situations in which anaerobic respiration is useful.
- Compare anaerobic respiration in yeasts and humans.
- Compare the total amount of ATP made from anaerobic and aerobic respiration.
- State the location of aerobic respiration.
- Outline how anaerobic respiration in yeast is used in baking.
- Outline how anaerobic respiration in yeast is used in ethanol production.
- State the condition in which humans would perform anaerobic respiration.
- Outline production of lactate in humans during anaerobic respiration.
- Outline the use of a respirometer to measure cellular respiration rate.
- List ethical questions that must be considered before using animals in experiments.
Define Respiration
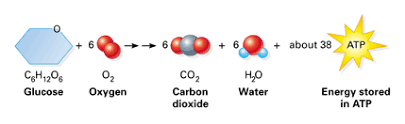
Respiration or Cellular respiration in plants is a catabolic process that involves the oxidation or breakdown of complex molecules into simple molecules. For example, the breakdown of glucose in presence of oxygen to release carbon-dioxide, water and energy.
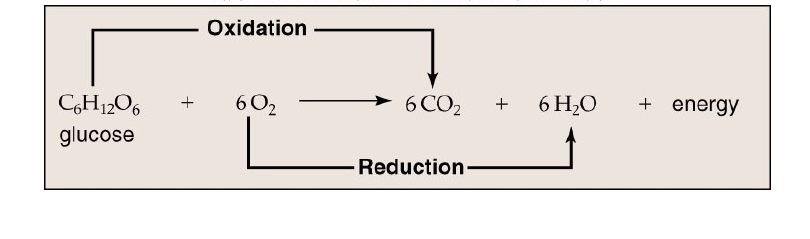
Fig.1. Breakdown of Glucose
The compounds that are oxidized during respiration are known as respiratory substrates, such as, glucose. The energy released during the process is in the form of ATP.
Glycolysis
The process of partial oxidation of glucose in presence of oxygen to form pyruvate is known as glycolysis. It occurs in all living cells in the cytoplasm. The scheme of glycolysis was given by Gustav Embden, Otto Meyerhof, and J. Parnas, So it is often referred to as the EMP pathway.
During glycolysis, one molecule of glucose breakdown to form two molecules of pyruvate. This glucose which undergo glycolysis comes from sucrose, the product of photosynthesis. Sucrose breakdown into glucose and fructose in presence of an enzyme invertase.
Steps of glycolysis are as follows:
- In the first step, glucose gets phosphorylated to form glucose-6-phosphate in presence of the enzyme hexokinase.
- This glucose 6-phosphate the isomerizes to form fructose-6-phosphate in presence of phosphoglucose isomerase.
- Fructose-6-phosphate used is converted into fructose-1,6-biphosphate using phosphofructokinase using ATP as the energy source.
- The fructose 1, 6-bisphosphate converted into dihydroxyacetone phosphate and 3-phosphoglyceraldehyde
- 3-phosphoglyceraldehyde is then converted into 1,3-biphosphoglycerate, to produce NADH.
- 1,3-biphosphoglycerate is converted into 3-phosphoglycerate with the formation of ATP in presence of phosphoglycerate kinase
- 3-phosphoglycerate is then converted into 2-phosphoglycerate in presence of phosphoglycerate mutase.
- 2-phosphoglycerate is converted into phosphoenol pyruvate in presence of enolase.
- Phosphoenol pyruvate is then finally converted into two molecules of pyruvate with the formation of ATP in presence of pyruvate kinase.
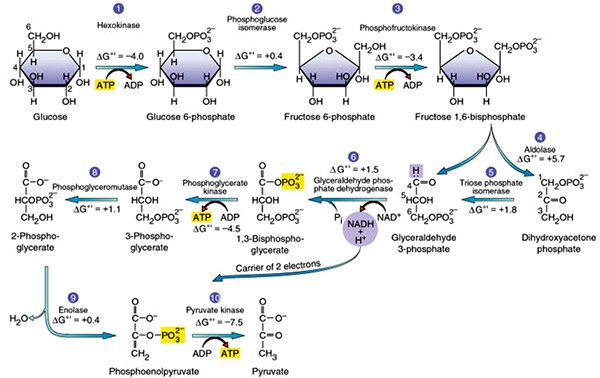
Fig.2. Pathway of Glycolysis
Significance of Glycolysis
It is used as a source of energy for almost all cells.
The product of glycolysis, that is, pyruvate has different fate. In the first case, pyruvate enter into Krebs cycle or citric acid cycle.
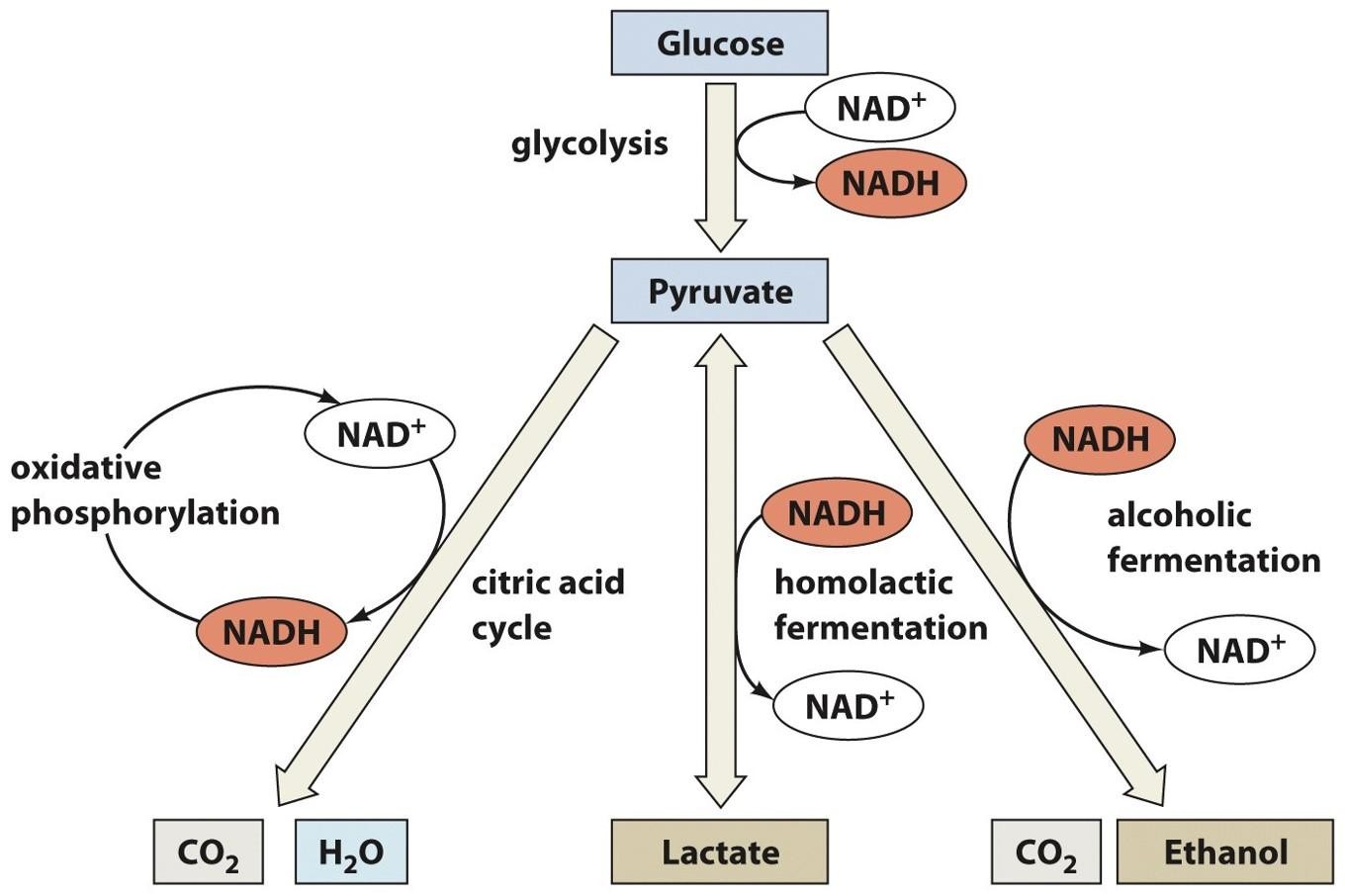
Fig.3. Fate of Pyruvate
In the second case, it can undergo homolactic fermentation to form lactate. Thirdly, it can undergo alcoholic fermentation to form ethanol and carbon-dioxide.
Fermentation/Anaerobic Respiration in Plants
Incomplete oxidation of glucose in absence of oxygen is known as fermentation. The end products are carbon-dioxide and ethanol. During fermentation pyruvate is metabolized into different compounds via different processes as explained below-
- Ethanol fermentation or alcoholic fermentation is the process of formation of ethanol and carbon-dioxide. Ethanol fermentation converts sugars such as glucose, fructose, sucrose into ethanol and carbon-dioxide. This process is used in the formation of alcoholic beverages.

Fig.4. Alcoholic Fermentation
- Lactic acid fermentation refers to the formation of lactic acid. There are two types of lactic acid fermentation-homolactic fermentation and heterolactic fermentation. Homolactic fermentation involves the formation of lactic acid only whereas heterolactic fermentation involves formation of lactic acid as well as other acids and alcohols. Lactic acid fermentation occurs in bacterial cells as well as in muscles during physical work such as exercise.
Krebs Cycle
The product of glycolysis, that is, pyruvate is transported from the cytoplasm into the mitochondria. Pyruvate enters the mitochondrial matrix where oxidative decarboxylation of pyruvate yield acetyl coenzyme A.
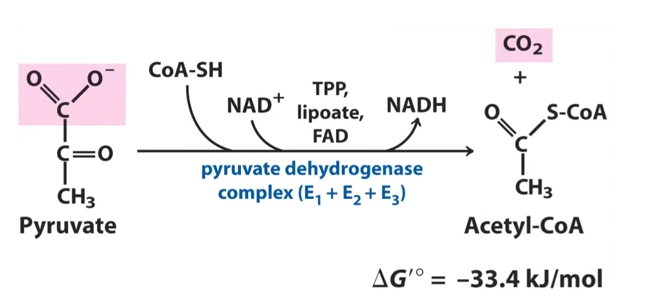
Fig.5.Reaction catalyzed by pyruvate dehydrogenase
Pyruvate dehydrogenase is a complex enzyme made up of three subunits E1, E2 and E3. These three subunits together form a complex. There are different cofactors that are involved during the reaction such as NAD+, FAD, TPP and lipoate.
This acetyl co-enzyme formed enters a cyclic process known as Krebs cycle or tricarboxylic acid cycle or citric acid cycle.
- The citric acid cycle starts with the condensation of acetyl co-enzyme A with oxaloacetic acid to form citric acid in presence of an enzyme citrate synthase.
- Citrate is then isomerized to form isocitrate.
- Isocitrate then undergo two successive decarboxylation reactions to form alpha-ketoglutaric acid and the succinyl coenzyme A with the formation of one molecule of NADH
- Succinyl coenzyme A is converted into succinic acid to form one molecule of GTP. This is substrate level phosphorylation.
- Succinic acid or succinate is then converted into fumarate in presence of an enzyme succinate dehydrogenase
- Fumarate is then converted into malate in presence of an enzyme fumarase with the formation of FADH2
- Malate is the oxidized into oxaloacetic acid in presence of an enzyme malate dehydrogenase with the formation of NADH.
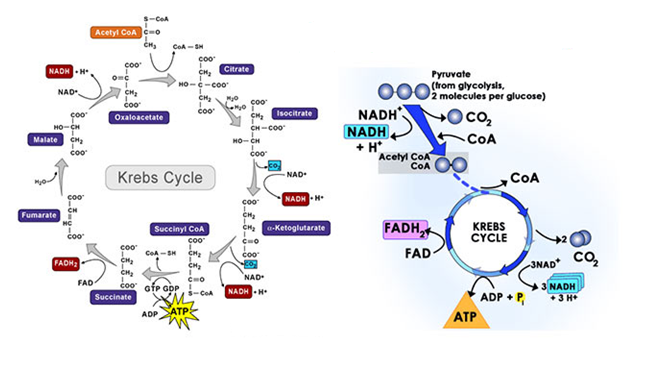
Fig.6. Tricarboxylic acid cycle
The overall reaction of Krebs cycle is given below:
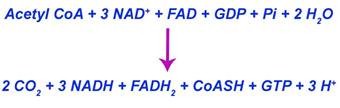
Significance of ATP
It is used to synthesize ATP from the oxidation of glucose, fatty acids and certain amino acids.
Electron Transport Chain (ETC) and Oxidative Phosphorylation
The products of glycolysis, that is, NADH and FADH2 are used to utilize or store energy. NADH and FADH2 are oxidized to release electrons. These electrons are passed to oxygen which results in the formation of water. It involves the different electron carriers that passes electrons which are finally received by oxygen.
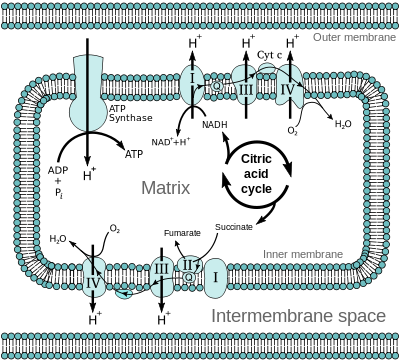
Fig.7. Electron transport chain
Electron carriers are in the form of different complexes. Electrons from NADH produced in the mitochondrial matrix during citric acid cycle are oxidized by a complex I known as NADH dehydrogenase. These electrons are then transferred to ubiquinone located within the inner membrane. Ubiquinone also receives reducing equivalents via FADH2 (complex II) that is generated during oxidation of succinate in the citric acid cycle. The reduced ubiquinone known as ubiquinol which is then oxidized with the transfer of electrons to cytochrome c via complex known as cytochrome bc1 complex (complex III). Cytochrome c is a small protein which acts as a mobile carrier for transfer of electrons between complex III and IV. Complex IV refers to cytochrome c oxidase complex containing cytochromes a and a3, and two copper centers.
The energy released via electron transport is involved in synthesis of ATP. The complex that helps in the formation of ATP is known as ATP synthase. ATP synthase is composed of two major components-F0 and F1.
The F1 is a peripheral membrane protein and is a site where ATP synthesis occurs from ADP and Pi. F0 is an integral membrane protein complex that forms the channel through which protons cross the inner membrane to form a protein gradient. For each ATP produced, 2H+
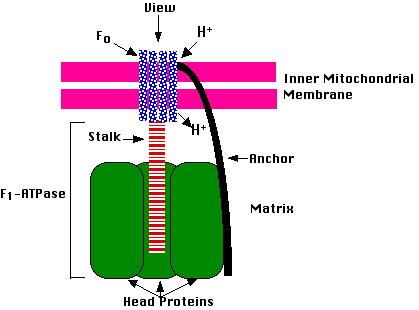
Fig.8. ATP synthase complex
passes through F0 from the intermembrane space to the matrix down the electrochemical proton gradient.
Significance of ETC
The electron transport chain is a system of molecules through which electronsare transferred to generate ATP. It has an important role in both photosynthesis and cellular respiration.
Amphibolic Pathway
Respiratory pathway is known as amphibolic pathway as it involves both anabolism and catabolism.
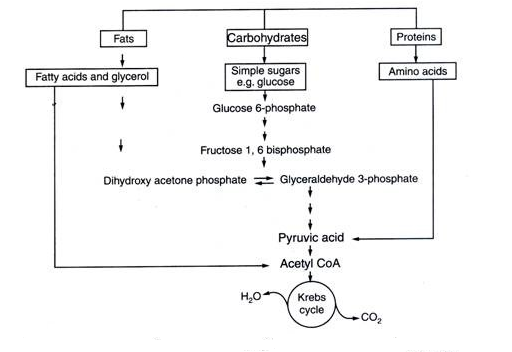
Fig.9. Interrelationship among different metabolic pathways to form carbon-dioxide and water
Respiratory Quotient
The ratio of the volume of CO2 evolved to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio. It is given by the formula:
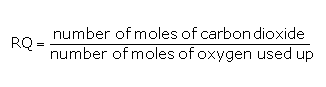
The respiratory quotient depends on the substrate that is used during respiration.
The RQ for different substrate is given below:
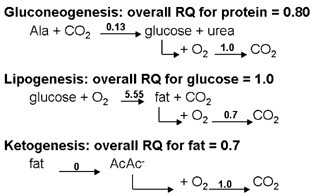
Fig.10. RQ for different substrates for respiration
Factors affecting Respiration in Plants
- Temperature affects the stability of different enzymes so it also affects the respiration. High temperature decreases the rate of respiration. Low temperature does not affect respiration significantly.
- Increase carbon-dioxide decreases the rate of respiration.
- Azide, cyanide are inhibitors of respiration.
- Young plant cells have high rate of respiration.
- More respiratory substrates more is the respiration.
Topic 2.8: cellular respiration
In the Cellular Respiration unit we will discuss aerobic and anaerobic cellular respiration. You will also learn how ATP is produced in the mitochondria.
The unit is planned to take 3 school days
Essential idea:
- Cell respiration supplies energy for the functions of life.
Nature of science:
- Assessing the ethics of scientific research—the use of invertebrates in respirometer experiments has ethical implications. (4.5)
- List ethical questions that must be considered before using animals in experiments.
2.8.U1 Cell respiration is the controlled release of energy from organic compounds to produce ATP.
- Define “cell respiration.”
- State the reaction for cellular respiration.
- State the types of organic compounds used in cellular respiration by animals and plants.
Cell respiration is the controlled release of energy from organic compounds to produce ATP. The main organic compound used for this process is carbohydrates (glucose), although lipids and proteins can also be digested
- organic compounds are broken down to release energy, which can be used in cells
- source of organic compounds broken down is food
- Carried out using enzymes carefully /controlled so that as much as possble is retained in a usable form
- Adenosine triphosphate – form is a chemical substance (ATP)
- Making ATP phosphate group is lined to ATP or ADP – energy to carry out the reaction comes from the breakdown of organic compounds
There are two main types of cell respiration:
- Anaerobic respiration involves the partial breakdown of glucose in the cytosol for a small yield of ATP
- Aerobic respiration utilises oxygen to completely break down glucose in the mitochondria for a larger ATP yield
2.8.U2 ATP from cell respiration is immediately available as a source of energy in the cell.
- State three example uses of cellular energy.
- Outline energy transfer in the formation and use of ATP.
- State three reasons why cellular respiration must be continuously performed by all cells.
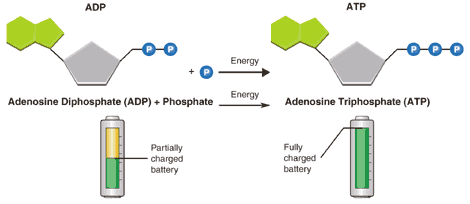
ATP (adenosine triphosphate) is a high energy molecule that functions as an immediate source of power for cell processes
- Used to synthesizing large molecules like DNA, RNA, and proteins
- Pumping molecules or ions across membranes by active transport
- Moving things within a cell, like chromosomes, vesicles, muscles cells the protein fibres
- The energy is immediately available
- Released by splitting ATP into ADP and phosphate –> can be reconverted back to ATP through cell respiration.
- One molecule of ATP contains three covalently linked phosphate groups – which store potential energy in their bonds
- When ATP is hydrolysed (to form ADP + Pi) the energy stored in the phosphate bond is released to be used by the cell
- Cell respiration uses energy stored in organic molecules to regenerate ATP from ADP + Pi (via oxidation)Most energy is converted to heat within a cell – no reusable for cell activities/ eventually lost to the environment
- Reason way they need ATP because it’s continual
2.8.U3 Anaerobic cell respiration gives a small yield of ATP from glucose.
- Define “anaerobic respiration”
- List three situations in which anaerobic respiration is useful.
- Compare anaerobic respiration in yeasts and humans.
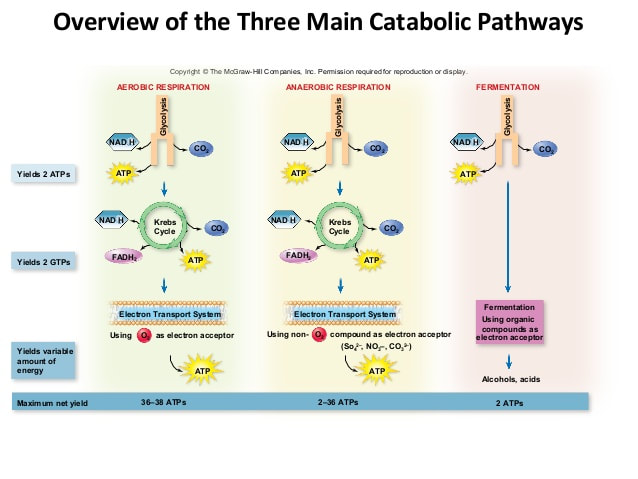
Both anaerobic and aerobic respiration pathways begin with the anaerobic breakdown of glucose in the cytosol by glycolysis
- glucose is broken down in anaerobic cell respiration without oxygen
- glycolysis breaks down glucose (6-C) into two molecules of pyruvate (3C), hydrogen carriers (NADH) from an oxidised precursor (NAD+)ATP yield is small but can be produced quickly (net gain of 2 molecules)
- when a short by fast burst of ATP production is needed
- when oxygen supplies run out in respiring cells
- in environments that are deficient in oxygen
- n animals, the pyruvate is converted into lactic acid (or lactate)
In plants and yeasts, the pyruvate is converted into ethanol and carbon dioxide
2.8.U4 Aerobic cell respiration requires oxygen and gives a large yield of ATP from glucose.
- Compare the total amount of ATP made from anaerobic and aerobic respiration.
- State the location of aerobic respiration.
if there’s oxygen then glucose can be more fully broken down to release a greater quantity of energy than in anaerobic cell respiration
- ATP two molecules per glucose in anaerobic cell respiration
- more then 30 per glucose in aerobic cell respiration
- Multiple chemical reactions – dioxide and water are produced
- eukaryotic cell- this happens inside the mitochondrion
2.8.A1 Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking.
- Outline how anaerobic respiration in yeast is used in baking.
- Outline how anaerobic respiration in yeast is used in ethanol production.
2.8.A2 Lactate production in humans when anaerobic respiration is used to maximize the power of muscle contractions.
- State the condition in which humans would perform anaerobic respiration.
- Outline production of lactate in humans during anaerobic respiration.
Aerobic cell respiration requires oxygen and gives a large yield of ATP from glucose. Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking. Lactate production in humans when anaerobic respiration is used to maximize the power of muscle contractions
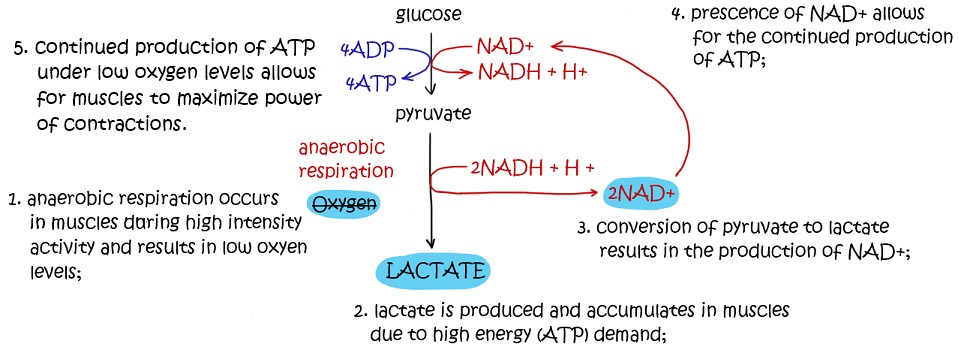
Muscle contractions require the expenditure of high amounts of energy and thus require high levels of ATP. When exercising at high intensity, the cells’ energy demands will exceed what the available levels of O2 can supply aerobically. Therefore the body will begin breaking down glucose anaerobically to maximise ATP production. This will result in an increase in the production of lactic acid, which leads to muscle fatigue.
When the individual stops exercising, oxygen levels will increase and lactate will be converted back to pyruvate. Although carbohydrates, lipids and proteins can all be consumed as energy sources, only carbohydrates will typically undergo anaerobic respiration
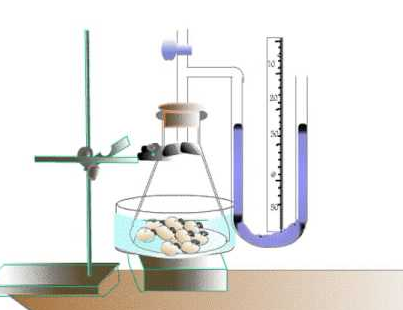
2.8.S1 Analysis of results from experiments involving measurement of respiration rates in germinating seeds or invertebrates using a respirometer.
- Outline the use of a respirometer to measure cellular respiration rate.
Carbon dioxide production can be measured with a data logger or by pH changes if the specimen is immersed in water
When an alkali is included to absorb CO2, oxygen consumption can be measured as a change in pressure within the system. The pressure change can be detected with a data logger or via use of a U-tube manometer
Factors which may affect respiration rates include temperature, hydration, light (plants), age and activity levels
An increase in carbon dioxide levels will indicate an increase in respiration (CO2 is a product of aerobic respiration)
A decrease in oxygen levels will indicate an increase in respiration (O2 is a requirement for aerobic respiration)
Cell respiration
8.1.1 State that oxidation involves the loss of electrons from an element, whereas reduction involves a gain of electrons; and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen.
Oxidation involves the loss of electrons from an element, whereas reduction involves the gain of electrons and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen.
8.1.2 Outline the process of glycolysis, including phosphorylation, lysis, oxidation and ATP formation.
Step 1 – Glucose is phosphorylated. Two phosphate groups are added to glucose to form hexose biphosphate. These two phosphate groups are provided by two molecules of ATP.
Step 2 – Lysis of hexose biphosphate. Hexose biphosphate splits into two molecules of triose phosphate.
Step 3 – Each triose phosphate molecules is oxidised. Two atoms of hydrogen are removed from each molecule. The energy released by the oxidation is used to add another phosphate group to each molecule. This will result in two 3-carbon compounds, each carrying two phosphate groups. NAD+ is the hydrogen carrier that accepts the hydrogen atoms lost from each triose phosphate molecule.
Step 4 – Two pyruvate molecules are formed by removing two phosphate groups from each molecule. These phosphate groups are given to ADP molecules and form ATP.
Glycolysis occurs in the cytoplasm of cells. Two ATP molecules are used and 4 ATP molecules are produced. Therefore there is a net yield of two ATP molecules. Also, two NAD+ are converted into NADH + H+ during glycolysis.
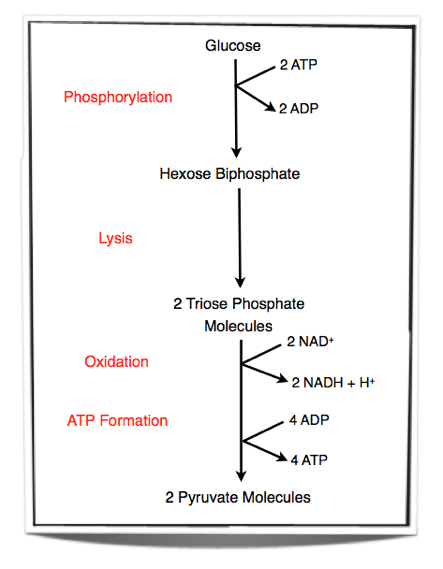
Figure 8.1.1 – Steps in glycolysis
8.1.3 Draw and label a diagram showing the structure of a mitochondrion as seen in electron micrographs.
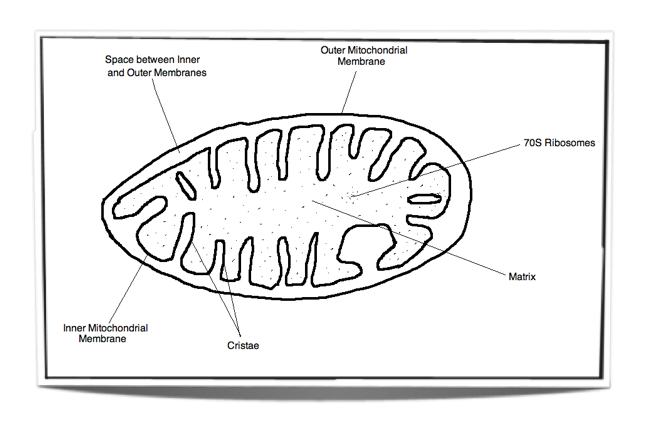
Figure 8.1.2 – Labelled diagram of a mitochondrion
8.1.4 Explain aerobic respiration, including the link reaction, the Krebs cycle, the role of NADH + H+, the electron transport chain and the role of oxygen.
Aerobic Respiration
Glycolysis can take place without oxygen. This forms the anaerobic part of cell respiration and therefore is called anaerobic cell respiration. However, the pyruvate produced from glycolysis cannot be oxidised further without the presence of oxygen. The oxidisation of the pyruvate forms part of the aerobic respiration and therefore is called aerobic cell respiration. Aerobic respiration occurs in the mitochondria of cells. The first reaction to take place is the link reaction.
The Link Reaction
Mitochondria in cells take up the pyruvate which is formed from glycolysis in the cytoplasm. Once the pyruvate is in the mitochondrion, enzymes within the matrix of the mitochondrion remove hydrogen and carbon dioxide from the pyruvate. This is called oxidation (removal of hydrogen or addition of oxygen) and decarboxylation (removal of carbon dioxide). Therefore, the process is called oxidative decarboxylation. The hydrogen removed is accepted by NAD+. The link reaction results in the formation of an acetyl group. This acetyl group is then accepted by CoA and forms acetyl CoA.
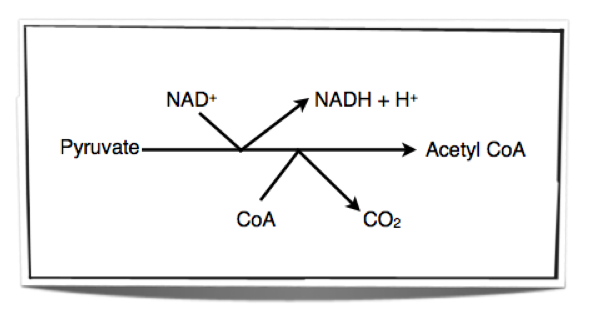
Figure 8.1.3 – The link reaction
The Krebs Cycle
Step 1 – In the first stage of the Krebs cycle, the acetyl group from acetyl CoA is transferred to a four carbon compound. This forms a six carbon compound.
Step 2 – This six carbon compound then undergoes decarboxylation (CO2 is removed) and oxidation (hydrogen is removed) to form a five carbon compound. The hydrogen is accepted by NAD+ and forms NADH + H+.
Step 3 – The five carbon compound undergoes decarboxylation and oxidation (hydrogen is removed) again to form a four carbon compound. The hydrogen is accepted by NAD+ and forms NADH + H+.
Step 4 – The four carbon compound then undergoes substrate-level phosphorylation and during this reaction it produces ATP. Oxidation also occurs twice (2 hydrogens are removed). The one hydrogen is accepted by NAD+ and forms NADH + H+. The other is accepted by FAD and forms FADH2. The four carbon compound is then ready to accept a new acetyl group and the cycle is repeated.
The carbon dioxide that is removed in these reactions is a waste product and is excreted from the body. The oxidations release energy which is then stored by the carriers when they accept the hydrogen. This energy is then later on used by the electron transport chain to produce ATP.
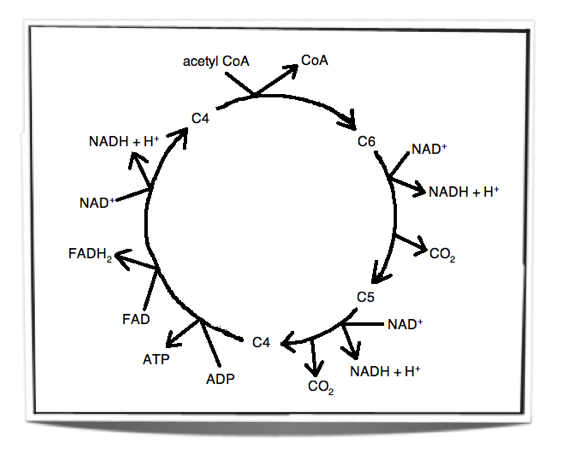
Figure 8.1.4 – Krebs cycle
To summarise:
Carbon dioxide is removed in two reactions
Hydrogen is removed in 4 reactions
NAD+ accepts the hydrogen in 3 reactions
FAD accepts the hydrogen in 1 reaction
ATP is produced in one of the reactions
The Electron Transport Chain
Inside the inner membrane of the mitochondria there is a chain of electron carriers. This chain is called the electron transport chain. Electrons from the oxidative reactions in the earlier stages of cell respiration pass along the chain. NADH donates two electrons to the first carrier in the chain. These two electrons pass along the chain and release energy from one carrier to the next. At three locations along the chain, enough energy is released to produce ATP via ATP synthase. ATP synthase is an enzyme that is also found in the inner mitochondrial membrane. FADH2 also donates electrons but at a later stage than NADH. Also, enough energy is released at only two locations along the chain by electrons from FADH2. The ATP production relies on energy release by oxidation and it is therefore called oxidative phosphorylation.
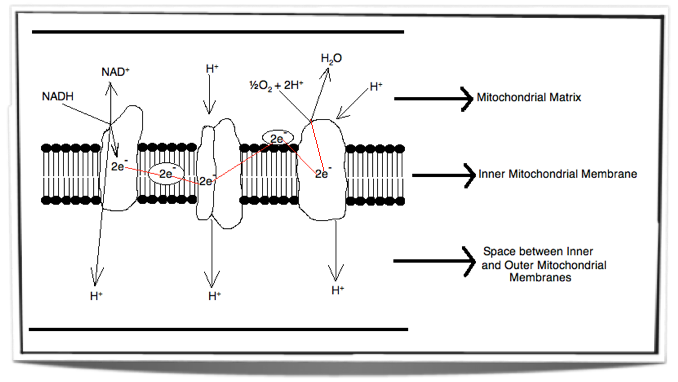
Figure 8.1.5 – Electron transport chain
The Role of Oxygen
Oxygen is important for cell respiration as at the end of the electron transport chain, the electrons are donated to oxygen. This occurs in the matrix at the surface of the inner membrane. At the same time oxygen binds with hydrogen ions and forms water.
If there is no oxygen then electrons can no longer pass through the electron transport chain and NADH + H+ can no longer be reconverted into NAD+. Eventually NAD+ in the mitochondrion runs out and therefore the link reaction and Krebs cycle no longer take place.
8.1.5 Explain oxidative phosphorylation in terms of chemiosmosis.
When electrons pass through the electron transport chain they release energy. This energy is then used to pump protons (H+) from the matrix across the inner mitochondrial membrane and into the space between the inner and outer mitochondrial membranes. The space between the inner and outer membranes has a small volume and therefore as the protons move across they create a concentration gradient very quickly. This process is called chemiosmosis. There is now a high concentration of protons in the space between the inner and outer membranes and a low concentration of protons in the matrix.
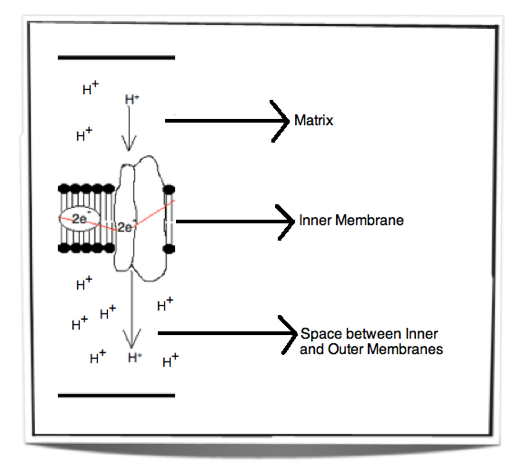
Figure 8.1.6 – Chemiosmosis
Figure 8.1.6 shows the movement of protons from the matrix into the space between the inner and outer membranes. This creates a concentration gradient. The energy used to pump these protons across the inner membrane comes from the energy released by the electrons passing through the electron transport chain.
The protons then move down the concentration gradient from the space between the inner and outer membranes back into the matrix. However, they can only move back across via an enzyme embedded in the inner membrane. This enzyme is called ATP synthase. The protons are transported back into the matrix through the channels of ATP synthase and as they do so they release energy. This energy is then used by ATP synthase to convert ADP into ATP. Since the electrons come from previous oxidation reactions of cell respiration and the ATP synthase catalyses the phosphorylation of ADP into ATP, this process is called oxidative phosphorylation. Chemiosmosis is necessary for oxidative phosphorylation to work.
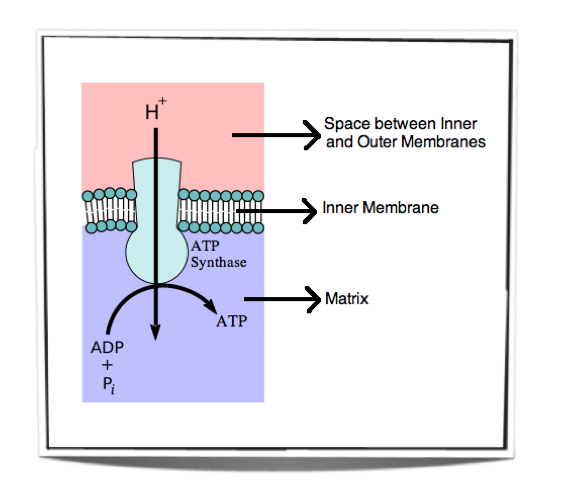
Figure 8.1.7 – Oxidative phosphorylation
Figure 8.1.7 shows the movement of protons down their concentration gradient. They can only travel through the inner membrane via ATP synthase and as they do so they release energy. This energy is used by ATP synthase to convert ADP into ATP.
8.1.6 Explain the relationship between the structure of the mitochondrion and its function.
Matrix: Watery substance that contains ribosomes and many enzymes. These enzymes are vital for the link reaction and the Krebs cycle.
Inner membrane: The electron transport chain and ATP synthase are found in this membrane. These are vital for oxidative phosphorylation.
Space between inner and outer membranes: Small volume space into which protons are pumped into. Due to its small volume, a high concentration gradient can be reached very quickly. This is vital for chemiosmosis.
Outer membrane: This membrane separates the contents of the mitochondrion from the rest of the cell. It creates a good environment for cell respiration.
Cristae: These tubular projections of the inner membrane increase the surface area for oxidative phosphorylation.
