- IB Style Question Banks with Solution
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
4.4 Climate Change
Essential Idea:
Concentrations of gases in the atmosphere affect climates experienced at the Earth’s surface
Understandings:
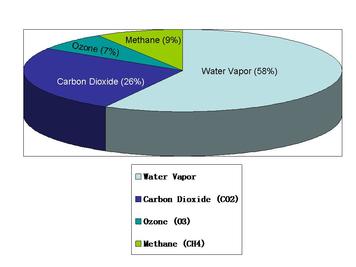
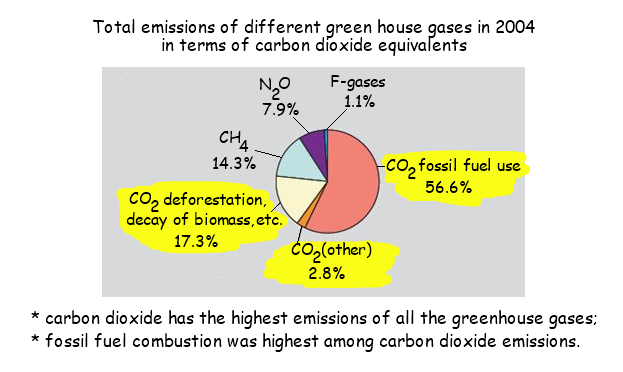
- Carbon dioxide and water vapour are the most significant greenhouse gases
- Other gases including methane and nitrogen oxides have less impact
- The impact of a gas depends on its ability to absorb long wave radiation as well as on its concentration in the atmosphere
- The warmed Earth emits longer wavelength radiation (heat)
- Longer wave radiation is absorbed by greenhouse gases that retain the heat in the atmosphere
- Global temperatures and climate patterns are influenced by concentrations of greenhouse gases
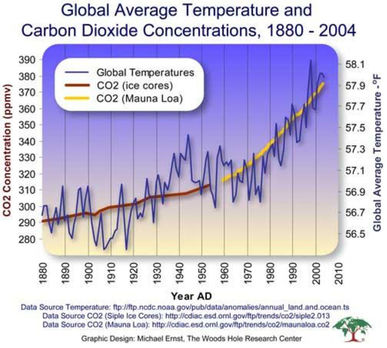
- There is a correlation between rising atmospheric concentrations of carbon dioxide since the start of the industrial revolution 200 years ago and average global temperatures
- Recent increases in atmospheric carbon dioxide are largely due to increases in the combustion of fossilised organic matter
Applications:
- Threats to coral reefs from increasing concentrations of dissolved carbon dioxide
- Correlations between global temperatures and carbon dioxide concentrations on Earth
- Evaluating claims that human activities are not causing climate change
- State the sources of CO2 and water vapor in the atmosphere.
- Outline the mechanism by which greenhouse gases trap heat in the atmosphere.
- State the sources of methane and NO gases in the atmosphere.
- State two factors that determine the warming impact of a greenhouse gas.
- State two variables that determine the concentration of a gas in the atmosphere.
- Compare the impact of atmospheric methane to CO2.
- State how long water, methane and CO2 remain in the atmosphere, on average.
- State that the Earth absorbs short-wave energy from the sun and re-emits longer wavelengths.
- Compare wavelengths of UV, visible and infrared radiation.
- Explain the greenhouse effect, with reference to short wave radiation from the sun, long wave radiation from the Earth and the effects of ozone and greenhouse gases.
- Explain why water vapor, CO2, methane and NO are greenhouse gases.
- Explain why atmospheric CO2 concentration would logically impact global temperatures.
- Outline the effect of global temperature on climate, specifically location and frequency of of rain and frequency of severe storms.
- State the atmospheric CO2 concentration prior to the industrial revolution.
- Outline the impact of the industrial revolution on atmospheric CO2 concentration.
- Describe the correlation between atmospheric CO2 concentrations since the industrial revolution and global temperatures.
- Explain why industrial revolution would increase atmospheric CO2 concentrations.
- Explain how historical temperature data has been collected.
- Using ice core data, outline the correlation between atmospheric CO2 concentration and global temperatures.
- Outline three reasons why there is vigorous debate around the claim that human activities are causing climate change.
- Outline the effect of atmospheric CO2 concentration on ocean pH.
- Describe the impact of lower ocean pH on animals that make skeletons from calcium carbonate.
- Outline ways by which claims can be evaluated for truth.
4.4 climate change
In the Climate Change unit, we take a look at how certain gases, the most important of which are carbon dioxide and water vapour, enable the atmosphere to retain heat. Without these gases in the atmosphere, the Earth’s temperature would be too low to support life. We will also look at how the warming effect of these gases known as the greenhouse effect caused in a similar way to the warming of a greenhouse.
This unit will last 3 school days
Essential idea:
- Concentrations of gases in the atmosphere affect climates experienced at the Earth’s surface.
Nature of science:
- Assessing claims—assessment of the claims that human activities are producing climate change. (5.2)
- Outline ways by which claims can be evaluated for truth.
4.4.U 1 Carbon dioxide and water vapour are the most significant greenhouse gases.
- State the sources of CO2 and water vapor in the atmosphere.
- Outline the mechanism by which greenhouse gases trap heat in the atmosphere.
Greenhouse gases absorb and emit long-wave (infrared) radiation, thereby trapping and holding heat within the atmosphere
- Greenhouse gases collectively make up less than 1% of the Earth’s atmosphere
- Water vapour is created via evaporation of water bodies (e.g. oceans) and transpiration – it is removed via precipitation (rain)
- Carbon dioxide is made by cell respiration and burning fossil fuels – it is removed via photosynthesis and absorption by oceans
U 4.4.U2 Other gases including methane and nitrogen oxides have less impact.
- State the sources of methane and NO gases in the atmosphere.
Methane has the third greatest impact on the greenhouse effect. It is emitted from marches, other water-logged habitats and from landfill sites containing organic wastes.
Nitrous oxide, which is another significant greenhouse gas is released naturally by bacteria in some habitats and also by agriculture and vehicle exhaust.
All the greenhouse gases together make up less than 1% of the earth’s atmosphere
4.4.U3 The impact of a gas depends on its ability to absorb long wave radiation as well as on its concentration in the atmosphere.
- State two factors that determine the warming impact of a greenhouse gas.
- State two variables that determine the concentration of a gas in the atmosphere.
- Compare the impact of atmospheric methane to CO2.
- State how long water, methane and CO2 remain in the atmosphere, on average.
The two factors that determine how much of an influence a gas will have on the greenhouse effect are
- The ability of the gas to absorb long-wave radiation (heat)
- The concentration of the gas in the atmosphere
Methane actually has the ability to cause much more warming per molecule than carbon dioxide; however, there is a much lower concentration of methane in the atmosphere
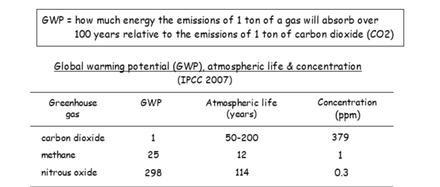
Each greenhouse gas (GHG) has different effects on warming the Earth. It will depend on the GHG’s ability to absorb enerty, and how long it remains in the atmosphere.
Global Warming Potential (GWP) is been developed to make comparisons of the global warming impacts of different GHGs.
GWPs provide a common unit of measure allowing for the adding up emissions estimates of different gasses
4.4.U4 The warmed Earth emits longer wavelength radiation (heat).
- State that the Earth absorbs short-wave energy from the sun and re-emits longer wavelengths.
- Compare wavelengths of UV, visible and infrared radiation.
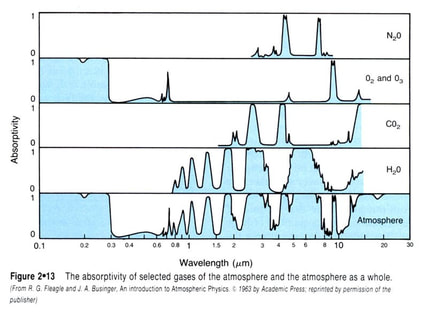
Some of the light is converted into heat, which in turn warms the surface of the earth (the air, mountains and water). This heat (longer wavelengths) radiates off the earth back towards the atmosphere. The peak wavelength for infrared is 10,000 nm, while the peak wavelength of solar radiation is 400 nm
4.4.U5 Longer wave radiation is absorbed by greenhouse gases that retain the heat in the atmosphere
- Explain the greenhouse effect, with reference to short wave radiation from the sun, long wave radiation from the Earth and the effects of ozone and greenhouse gases.
- Explain why water vapor, CO2, methane and NO are greenhouse gases.
70-75% of the solar radiation reaches the earth’s surface, with a high percentage of this radiation being converted into heat
As the infrared radiation is reflected back off the earth, a large percentage of this heat is captured by the greenhouse gases in the atmosphere. This energy is re-emitted, thus heating up the earth’s atmosphere. This effect is called global warming.
The ability of the earth’s surface to reflect light is called the albedo effect. Light colored and white objects such as snow and ice, have a high albedo and therefore little light is absorbed and less heat is produced. Black and dark colored objects like asphalt and pavement have a low albedo, and therefore absorb more light and produce more heat.
With the spread of urban cities and areas, a greater amount of heat is being produced
4.4.U6 Global temperatures and climate patterns are influenced by concentrations of greenhouse gases.
- Explain why atmospheric CO2 concentration would logically impact global temperatures.
- Outline the effect of global temperature on climate, specifically location and frequency of of rain and frequency of severe storms.
Climate refers to the patterns of temperature and precipitation that occur over long periods of time. Climate changes over thousands or millions of years. Climatologists and palaeoclimatologists collect and study data about atmospheric conditions in recent decades and from the distant past in order to infer what the climate was like thousands to millions of years ago.
Since greenhouse gases cause the earth to retain heat, one can infer that the more greenhouse gas there is in the atmosphere, the warmer the earth will be. This does not mean that the amount of greenhouse gas is the only reason for the earth warming and cooling; however, there is a correlation between the earth’s temperature and the amount of greenhouse gas. Other factors such as the cycles in the Earth’s orbit around the sun, variations in the amount of solar radiation due to sunspot activity, past volcanic activity, and changes or oscillations in ocean currents
Even if greenhouse gases aren’t the only factor in the rise of the Earth’s temperature, if the Earth heats up even a few degrees, it will have profound effects on the climate patterns
(IPCC 2013: http//www.ipcc.ch/report/or5/wg1/
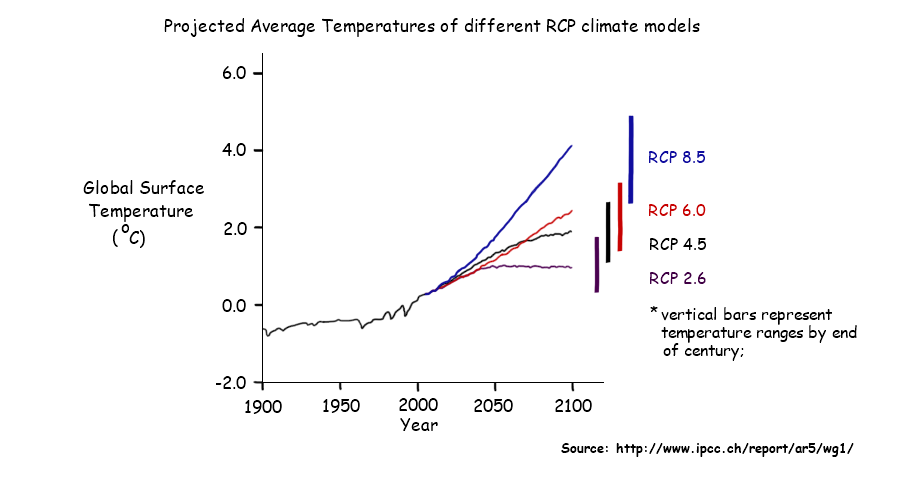
- Increases in average global temperatures projected within range of -17.5 degree C to -13 degree C by 2010 with likely increase to be at least -16.3
- Global average temperature is expected to be at least twice as wram in the next 100 years as it has during the last 100 years
- Ground-level air temperatures are expected to warm more rapidly over land than oceans
- Larger temperature increases projected to be greater than the global average in some parts of the world.
(IPCC 2013: http//www.ipcc.ch/report/or5/wg1/
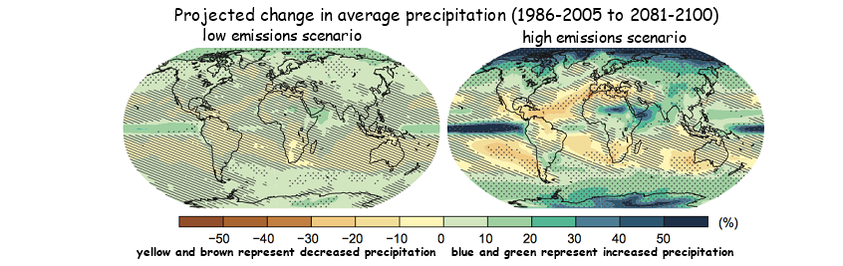
- Global average annual precipitation expected to increase to end of century, however, changes the amount and intensity of precipitation will vary significantly by region
- On average, an increase in the intensity of precipitation events, more pronounced in tropical and high-latitude regions
- Increase in wind strenghts and amount of precipitation associated with tropical storms
- Projected annual average precipitation to increase in some areas and decrease in others
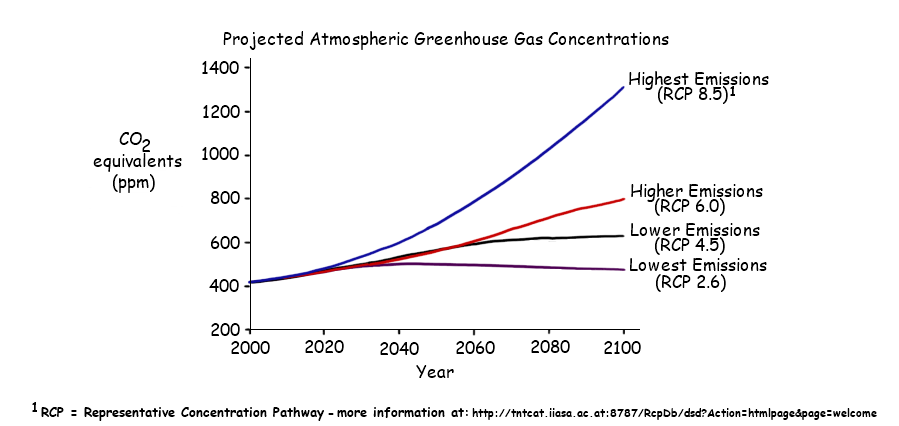
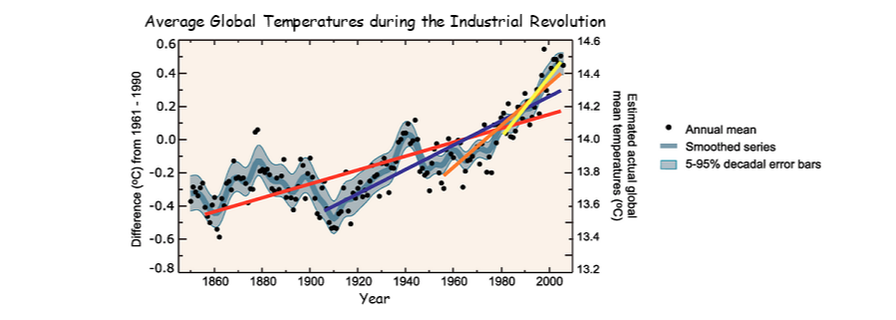
4.4.U7 There is a correlation between rising atmospheric concentrations of carbon dioxide since the start of the industrial revolution 200 years ago and average global temperatures
- State the atmospheric CO2 concentration prior to the industrial revolution.
- Outline the impact of the industrial revolution on atmospheric CO2 concentration.
- Describe the correlation between atmospheric CO2 concentrations since the industrial revolution and global temperatures.
Graph also shows the rate of temperature increase (the steepness of the slope: red, blue, orange, becomes larger as more of the world became industrialized
4.4.U8 Recent increases in atmospheric carbon dioxide are largely due to increases in the combustion of fossilized organic matter.
- Explain why industrial revolution would increase atmospheric CO2 concentrations.
Another source of carbon dioxide is the deforestation through burning large tracks of land and heating homes with fossil fuels, such as natural gas. Humans demand for meat has led to large numbers of cattle, which is responsible for releasing methane into the atmosphere, which is changed into carbon dioxide. As the human population increases and countries become more industrialized, human production of greenhouse gas, shows no sign of slowing down
4.4.S1 Threats to coral reefs from increasing concentrations of dissolved carbon dioxide.
- Explain how historical temperature data has been collected.
- Using ice core data, outline the correlation between atmospheric CO2 concentration and global temperatures.
Ocean acidification is the ongoing decrease in the pH of the Earth’s oceans, caused by the uptake of carbon dioxide from the earth’s atmosphere. Over 500 billion tons of CO2 released by humans since the start of the industrial revolution have been dissolved in the oceans. pH of surface layers of the earth’s oceans in the late 18th century ≈ 8.1179 currently ≈ 8.069, which represents about a 30% acidification.
Reef-building corals that use calcium carbonate in their exoskeletons need to absorb carbonate ions from seawater.
The concentration of carbonate ions is low in seawater because they are not very soluble. Dissolved CO2 makes the carbonate concentration even lower as a result of some interrelated chemical reactions: CO2 + H2O <–> H2CO3 <–> H+ + HCO3− <–> H+ + CO32 –
If the carbonate ions concentrations drop it is more difficult for reef-building corals to absorb these ions to make their exoskeletons. Also, if seawater ceases to be a saturated solution of carbonate ions, existing calcium carbonate tends to dissolve, so existing exoskeletons of reef-building corals are threatened.
Volcanic vents in the Gulf of Naples have been releasing carbon dioxide into the water for thousands of years, reducing the pH of the seawater. In this area of acidified water there are no corals, sea urchins or other animals that make their exoskeletons from calcium carbonate. In their place other organisms like invasive algae and sea grasses flourish. Unfortunately this could be the disheartening future for coral reefs around the world if carbon dioxide emissions continue to rise… Loss of tropical coral reefs to acidification could cost $1 trillion by 2100 in terms of lost shoreline protection and lost revenues for the tourism and food industries.
4.4.S2 Correlations between global temperatures and carbon dioxide concentrations on Earth.
- Outline three reasons why there is vigorous debate around the claim that human activities are causing climate change.
- Correlation does not imply causation
- Hundreds of years is not enough time to establish the validity of such a relationship
- To establish a causal relationship it must be demonstrated that the presumed cause precedes the presumed effect
- This relationship should be demonstrable over several cycles of increases and decreases in both of the parameters
4.4.S3 Evaluating claims that human activities are not causing climate change
- Outline the effect of atmospheric CO2 concentration on ocean pH.
- Describe the impact of lower ocean pH on animals that make skeletons from calcium carbonate.
Argument human activities not causing climate change
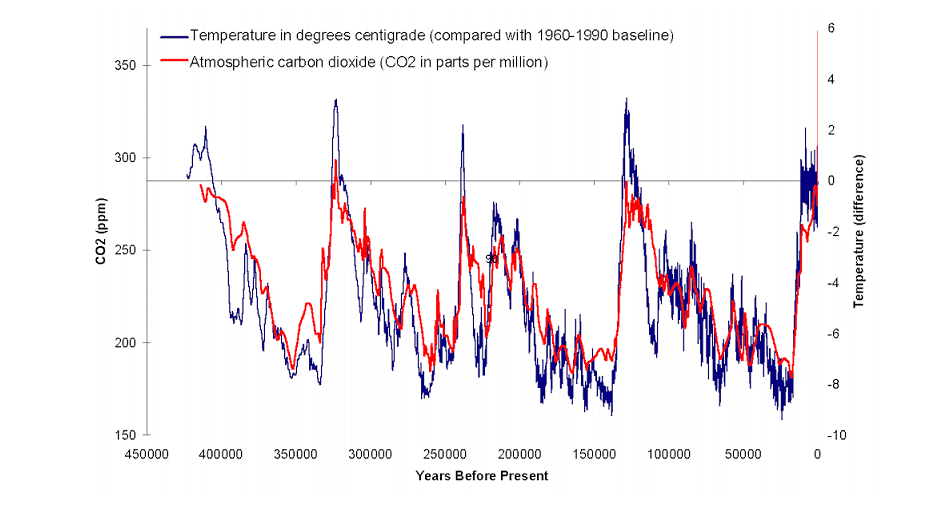
- Three glacial terminations and four glacial inceptions over the last half million years
- Noted that increases and decreases in atmospheric CO2 concentration not only did not precede the changes in air temperature, CO2 concentrations followed air temperatures
- CO2 levels followed temperatures by hundreds of years later
- Also noted times when CO2 level dropped and air temperature remained unchanged or actually rose
- Hence, the past half-million years provides absolutely no evidence ongoing rise in the CO2 levels will lead to significant global warming/climate change.
Environmental Issues
Table of Content
- Introduction to Environmental Issues
- Environmental Issues
- Air Pollution and its control
- Effect of air pollution
- Pollution caused by thermal power plant
- Prevention of air pollution: Ways to remove particulate matter
- Controlling Vehicular Pollution: A case study of Delhi
- Other parallel steps taken in Delhi
- New auto fuel policy to cut down vehicular pollution
- Laws passed by some countries to control Air Pollution
Introduction to Environmental Issues
Human population is expanding enormously; hence there is a great deal of interest for food, power, dress, streets, lodging, and vehicles and so on. These are applying a considerable measure of force on water, land air and other different assets. This prompts to pollution and debasement of nature and biodiversity that is a piece of it.
Environmental Issues
- Pollution is any undesirable changed in chemical, physical or organic attributes of water, air, soil or land.
- Pollutant: Any liquid, solid or gas discharged into nature in such an immense amounts, to the point that makes our surroundings unhealthy is called pollutant.
- Environment (protection) Act, 1986 to ensure and enhance the nature of our surroundings (water, air and soil)
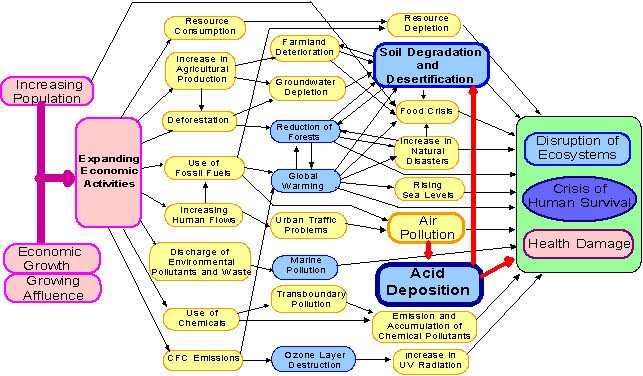
Fig: Environmental Issues
Air Pollution and Its Control
Effect of air pollution
- Cause harm to every single living life form
- Reduce development and yield of harvests
- Cause sudden death of plants
- Affects the respiratory arrangement of person
- Particulate estimate 2.5 micrometers or less are in charge of breathing and respiratory side effects like inflammations, irritation, and harm to the lungs and unexpected death.
Pollution caused by thermal power plant
- Sources of particulate matter: smelters, and thermal power plant
- These plants discharge vaporous air pollutant and particulate matter
- A safe gas discharged by these plants is Oxygen and Nitrogen

Fig: Air Pollution
Prevention of air pollution: ways to remove particulate matter
- Electrostatic Precipitator
- Extensively used to expel particulate matter in the fumes from a thermal power plant
- Electrode wires that are kept up at a few thousand volts, which create a corona that discharge electrons
- Electron binds together with particulate matter giving them a net negative charge
- The collecting plates that are positively charged draw in the charged dust molecule
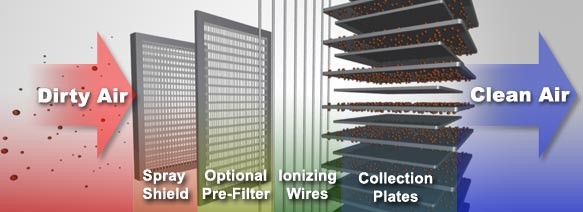
Fig: Electrostatic Precipitator
- Scrubber
- Removes gases like sulfur dioxide
- The fumes go through a splash of water or lime
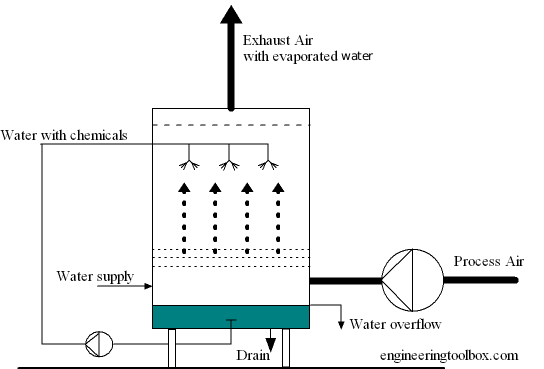
Fig: Scrubber Basics
- Methods to reduce vehicular pollution:
- The usage of lead-free petrol or diesel can decrease vehicular pollution.
- Catalytic converter:
- Having costly metals in particular platinum, rhodium, and palladium as the impetus.
- These metals decrease emanation of harmful gasses.
- The unburnt hydrocarbons are changed over into water and carbon dioxide.
- Nitric oxide and carbon monoxide are converted to nitrogen gas and carbon dioxide individually.
- Motor vehicle furnished with exhaust system ought to utilize unleaded petrol since lead present in the petrol tends to inactivate the impetus.
Controlling Vehicular pollution: A case study of Delhi
- Use of CNG (Compressed Natural Gas):
Advantages of CNG
- CNG blazes most proficiently
- Cannot be siphoned
- Very little remain unburnt
- CNG is less expensive than petrol and diesel
- Cannot be debased like petrol or diesel
Problem of use of CNG:
- Non-affirmation of continuous supply
- Difficulty in setting down pipelines to convey CNG
Other parallel steps taken in Delhi
- Phasing out old vehicles
- Use of unleaded petrol
- Use of low-sulfur petrol and diesel
- Use of exhaust system in vehicles
- Application of strict pollution level standards for vehicle
- Delhi: Odd Even Policy
- The dubious and highly discussed odd-even policy forced by the Delhi government to control vehicular pollution was presented by the Aam Aadmi Party government for a trial period of 15 days as a measure to tear into rising air pollution levels and smog in the national capital. As per the govern, autos with even-numbered enrollment plates will employ on even dates while those with odd tags will handle on odd dates. Bikes, vehicles that are driven by ladies, vehicles with differently- abled people and those of VIP, VVIP, for example, PM, President, Chief Justice, CJI and CMs of states and union regions were exempted by the run the show. Generally speaking, the rule was an accomplishment regarding decreasing blockage and movement on the streets, however, there has been no complete reply on whether pollution levels for sure descended amid the period. The state government, obviously, hailed the arrangement and said it prompted to an extraordinary decrease of PM 2.5 levels in the inside ranges of Delhi. Be that as it may, on a few days, pollution sheets introduced at a few places in the city demonstrated no critical lessening in pollution levels.
New auto fuel policy to cut down vehicular pollution
- Steadily diminishing the sulfur and sweet-smelling content in petrol and diesel fills.
- Euro-II standards
- Sulphur diminished to 350 ppm in diesel
- Sulphur diminished to 150 ppm in petrol
- Aromatic hydrocarbon to be diminished to 42%
- Up degree of vehicle motors
- Due to above strides taken by Delhi Govt. there is significant fall in CO2 and SO2 level somewhere around 1997 and 2005.
Laws passed by some countries to control Air Pollution
- Air Act (1981) Environment Act (1986)~ India
- Environmental Protection Act (1990) Environment Act (1995)_British Clean Air Act (1956)~UK
- Air Pollution Control Act (1955) Clean Air Act (1963,1970,1990)~US
- Environmental Promotion Act (1994) Environmental Compatibility Act (1994)~Austria.
Noise Pollution
- Undesirable abnormal state of sound is called Noise Pollution.
- Noise pollution is a noteworthy issue in India. The legislature of India has rules and regulations against sparklers and amplifiers, yet requirement is to a great degree lax. Awaaz (sound) Foundation is an Indian NGO attempting to control noise pollution from different sources through promotion, open intrigue case, mindfulness, and instructive campaigns since 2003. Despite expanded implementation and stringency of laws now being polished in urban regions, rustic zones are still influenced.
Harmful effect of noise pollution
- Psychological and physiological confusion in people
- Noise causes restlessness
- Altered breathing pattern
- High sound level, at least 150db may harm ear drums
- Increased heart rate
Prevention of Noise Pollution
- Utilization of sound permeable materials or by muting noise in enterprises
- Permissible sound levels of crackers
- Demarcation of horn free zones around healing facilities and schools
- Timings after which Loudspeakers can’t be played
Water Pollution and Its Control
Domestic sewage and industrial effluents
- A simple 0.1 percent polluting influences make residential sewage unfit for human utilize
- Sewage contains suspended salts like phosphates, nitrates, and different supplements, and organic compounds and poisonous metal particles.
- The measure of natural matter in water is evaluated by BOD (Biological Oxygen Demand).
- Biochemical oxygen demand: the measure of Oxygen required for oxidizing all natural matter that is present in one liter of water.
- Changes happen on release of sewage into the waterway.
- Micro-organisms that are required in the biodegradation of natural matter in the water body that is at the receiving end devour a considerable measure of oxygen, subsequently there is sharp decrease in broke up oxygen downstream from the purpose of release.
- There is a high mortality of fishes and other sea- living organisms due to low DO (Dissolved Oxygen).
- Presence of vast measure of supplements in water additionally causes exorbitant development of Planktonic (free skimming) green growth, known as Algal Bloom.
- Algal blossom gives unmistakable shadings and color to water bodies
- Drop down in the water quality and high mortality of fishes
- Some blossom framing algae are to a great degree dangerous to animals and human
- Water Hyacinth (Eichhornia crassipes), the world’s most tricky aquatic weed termed as ‘Terror of Bengal’.
- Introduced to India for their dazzling blossoms
- Excessive development causes hinders in conduits
- They develop copiously in eutrophic bodies of water
- Causes ecosystem imbalance and elements of water body
- Sewage associated with diseases:
- Sewage from hospitals and homes contain pathogenic microorganisms
- Discharge of such sewage without appropriate treatment causes ailments like cholera, dysentery, jaundice, typhoid and so on.
- Toxic heavy metals (defined as elements with density > 5g/cm3), released from:-
- Petroleum industry
- Metal extraction and processing
- Paper manufacturing
- Chemical manufacturing industries
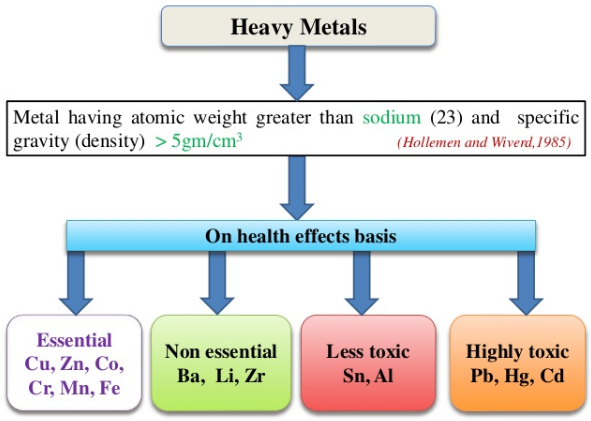
Fig. a: Heavy metals that cause toxicity

Fig. b: Symptoms of Heavy Metal Toxicity
- Biomagnifications: increment in toxicant concentration at progressive trophic level is called biological amplification or biomagnifications
- Toxic substance aggregated by a life form can’t be metabolized or discharged
- The aggregated poisonous material pass on to the following trophic level
- This process is notably known for mercury and DDT
- Bio magnification of DDT in Aquatic food chain
![]()
- Eutrophication: The procedure of supplement enhancement of water and subsequent loss of species diversity is alluded to as Eutrophication.

Fig: A eutrophic lake
- Natural Eutrophication:
- Streams depleting into the lake increment supplements like phosphorus and nitrogen
- Increase in levels of nutrients energizes the development of oceanic living beings
- Over hundreds of years, as sediment and debris get accumulated in the lake develops shallower and hotter
- Warm-water living beings overwhelm over that flourish in a cold domain
- Marsh plants flourish in the shallows and start to fill in the basin of the lake
- Eventually, the lake offers approach to vast masses of drifting plants (marsh), at long last changing over into land
- Cultural or Accelerated Eutrophication:
- Pollutants from man’s exercises like effluents from the businesses and homes can profoundly quicken the maturing process. This process is known as Cultural or Accelerated Eutrophication.
Causes
- Industrial and agricultural wastes and sewage
- Major contaminants are phosphates and nitrates
Effects
- Unsightly rubbish and disagreeable smell
- Robbing the disintegrated oxygen from water
- Inflow of pollutants inflow executes the fish
- Dead fish decomposition creates additional consumption of DO
- Finally, a lake can truly gag to death

Fig: Water Pollution
Thermal pollution

Fig: Thermal Pollution
Causes
Thermal waste waters that stream out of the power- generating units. For example thermal power plants.
Effects
- Thermal wastewater reduces or decreases the quantity of living being that are sensitized to high temperature.
- Enhance the development of fishes and plants fish in very cold territories yet simply in the wake of making harm to the indigenous vegetation.
A case study of integrated waste water treatment
- Wastewater including sewage can be dealt with in a coordinated way, by using a blend of fake and normal processes.
- It has been done nearby Arcata, in the northern bank of California.
- The treatment is done in two stages:
- The ordinary sedimentation, separating and chlorine treatment is given.
- The researcher built up a progression of six associated marshes more than 60 hectares of marshland.
- Appropriate bacteria, fungi, algae, and plants were seeded into this range, which kills, ingest, and acclimatize the pollutant
- The water moves through the marshes, it gets sanitized normally.
- The swamps additionally constitute a haven, with abnormal state of biodiversity as animals, fishes, and birds that now live there.
- A subjects group called Friends of the Arcata Marsh (FOAM) is in charge of the upkeep and protection of this venture.
Ecological Sanitation
- Ecological sanitation is a manageable framework for taking care of human excreta, utilizing dry, fertilizing the soil, toilets.
- This is a down to earth, a clean, proficient and financially savvy answer for discarding human waste.
- With this treating the soil strategy human excreta can be reused into a resource (as regular compost).
- ‘Eco San’ toilets are being utilized as a part of Srilanka and Kerala.
Solid Wastes
- Solid wastes allude to everything that goes out to waste.
- Municipal solid wastes are squanders from homes, workplaces, stores, schools, healing facilities and so forth. It involves paper, textile, food wastes, rubber, plastics, glass, leathers, metals, cowhides, and so on.
- Open clammy of these squanders serve as the reproducing ground for rats and flies.
- Sanitary landfills were received as substitute for open-smoldering dumps.
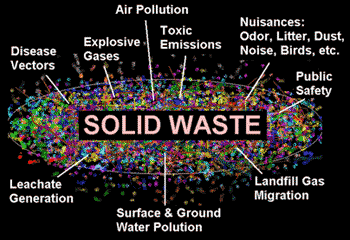
Fig: Image of environmental concerns associated with solid waste disposal
Sanitary Landfills
Wastes are dumped in a dejection or trench after compaction and secured with soil every day.
Disadvantages
- Shortage of space for immense garbage’s
- Danger of drainage of chemicals, dirtying the ground water assets
Solution to solid wastes
- Every single solid waste is arranged into three sorts:
- Bio-degradable
- Recyclable
- Non-biodegradable
- All the junk produce d is sorted first
- Recyclable material to be isolated and send for reuses
- Biodegradable squanders can be put into profound pits in the ground and be left for natural breakdown
- Only Non-biodegradable squanders are left and required to be arranged
Prevention
- The need to diminish our junk era ought to be a prime objective
- Reduction in use of plastics and utilization of eco-accommodating packaging
- Carrying cloth or other characteristic fiber carry sacks when we go shopping
- Denying polyethene packs
Case study of Remedy for Plastic wastes:
- Polyblend, a fine powder of reused adjusted plastic, was created by his organization
- Polyblend is blended with bitumen that is utilized to lay streets
- It builds the water repulsing property of bitumen, and expanded street life by an element of three
- The crude material utilized for polyblend is plastic film squander
Hospital Wastes
- Hospitals create risky squanders that contain disinfectants and other unsafe chemicals, furthermore pathogenic life forms.
- The utilization of incinerators is critical to discard wastes of healing facility.

Fig: Hospital Wastes
Electronic Wastes (E-wastes)
- Irreparable PCs and other electronic products are known as electronic wastes (e-wastes)
- E-Wastes are covered in landfills or burned
- Metals like copper, silicon, iron, nickel and gold are recouped amid reusing procedure of e-wastes
- Manual reusing process opens specialists to harmful substances display in e-wastes
- Recycling is the main answer for the treatment of e-wastes

Fig: Electronic Waste
Agro-Chemical and Their Effects
- Use of inorganic composts and pesticides has expanded complex for upgrading crop creation
- Pesticides, herbicides, fungicides and so forth, are by and large progressively utilized
- These are harmful to non-target creatures that are imperative parts of the soil ecosystem
- Increasing measures of manufactured composts causes eutrophication
Case study of organic farming:
- Integrated natural cultivating is a recurrent, zero waste strategy, where waste items from one process are cycled in as supplements for different procedures.
- Maximum use of asset and increment the proficiency of creation.
- He incorporates honey dairy management, bee keeping, journal administration, water gathering, treating the soil and farming in a chain of procedures, which bolster each other and permit a great degree efficient and practical example.
Advantages
- There is no need of utilization of chemical manures for products
- Cattle excreta are utilized as fertilizer.
- Crop waste used to make compost, which can be utilized as characteristic manure or can be utilized to produce natural gas for vitality needs.
Radioactive Wastes
- Nuclear vitality was hailed as a non-contaminating route for creating power.
- Later on, it was understood that it has two intense innate issue:
- Accidental spillage as happened in Three Mile Island and Chernobyl
- Safe transfer of radioactive wastes
- Radiation from radioactive waste causes transformation at a high rate.
- High measurements of atomic radiation is deadly, however, dosages in low amounts make hereditary disorders furthermore cause tumor.

Fig: Radioactive Wastes
Disposal of nuclear wastes
Storage of atomic waste, after adequate pre-treatment, ought to be done in reasonably protected holders covered inside the stones around 500 m far beneath the earth’s surface.
Green House Effect and Global Warming
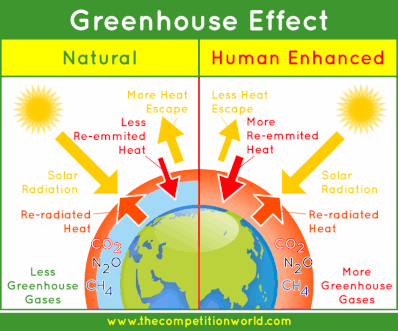
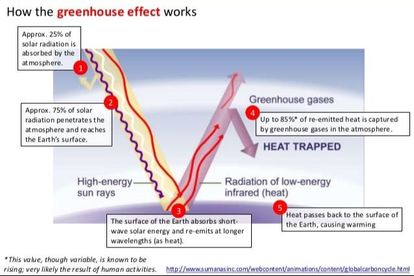
- The term “Greenhouse effect” has been gotten from a process that happens in the greenhouse.
- In a greenhouse, the glass board gives the light access, yet does not permit warmth to get away. In this manner, the greenhouse warms up, particularly like inside an automobile that has been stopped in the sun for a couple of hours.
- The greenhouse effect is a natural phenomenon that is in charge of warming of Earth’s surface and the environment.
- Without greenhouse effect the normal temperature at the surface of the earth would have been very cold – 18o C instead of the present normal of 15o C.
- Clouds and gasses reflect around one-fourth of the approaching sun-powered radiation and ingest some of it yet 50% of approaching sun based radiation falls on Earth’s surface warming it, while a little part is reflected back.
- Earth’s surface re-emanates warmth as infrared radiation, however, some piece of this does not escape into space as a result of climatic gasses (e.g. carbon dioxide, methane and so forth).
- The atom of these gasses emanates heat energy and a noteworthy piece of which again goes to Earth’s surface, therefore warming it up again.
- Carbon dioxide and methane – are generally known as greenhouse gasses in light of the fact that they are in charge of the greenhouse effect.
- Increase in the level of greenhouse gasses has prompted to impressive warming of Earth prompting to global warming or upgraded greenhouse effect.
- During the previous century, the temperature of Earth has expanded by 0.6o C.

Fig: Greenhouse Effect
Effect of Global Warming
- Deleterious changes in the earth and bringing about odd climatic changes (e.g. El Nino effect).
- Increased dissolving of polar ice tops and also of different spots like the Himalayan snow tops.
- Rise in ocean level that can submerge numerous beach front regions.
Control of Global Warming
- Reduce utilization of fossil fuel.
- Promoting afforestation program.
- Improving effectiveness of vitality use.
- Reducing deforestation.
- International activity to be taken to decrease emanation of greenhouse gasses.
- Slowing down development of the human population.
Ozone Depletion in the Stratosphere
- ‘Bad’ ozone shaped in the lower air (troposphere) that damages animals and plants.
- There is “good” ozone additionally; this ozone is found in the upper part of the environment called the stratosphere, and it goes about as a shield engrossing bright radiation from the sun.
- Measurement of the thickness of ozone layer is done in Dobson units (DU).
- Ozone (O3) gas is consistently shaped by the activity of UV beams on atomic oxygen, furthermore degraded into sub-atomic oxygen in the stratosphere.
- There ought to be appropriate balance between arrangement and debasement of ozone.
Ozone depletion
- Balance of ozone in the stratosphere is upset because of enhancement in degradation of the ozone layer by chlorofluorocarbons (CFCs).
- CFCs are widely used as refrigerants.
- CFCs released in the lower some portion of climate move upward and achieve stratosphere.
- In the stratosphere, UV beams follow up on CFCs and discharge dynamic Cl particles.
- Cl debases ozone discharging sub-atomic oxygen.
- Cl goes about as impetuses and not devoured amid chemical reactions.
- Whatever CFCs are added to the stratosphere, they have changeless and proceeding influences on Ozone levels.
- The depletion is stamped especially over the Antarctic district. This has brought about the formation of a vast territory of diminished ozone layer, generally called as the ozone hole.
Effects of UV rays
- UV radiations shorter than UV-B are totally consumed by Earth’s air if the ozone layer is in place.
- DNA and proteins of living life forms are harmed by UV beams as they conceivably retain it.
- The high vitality of UV beams softens the chemical bond in these atoms.
- UV – B harms DNA and mutations may happen.
- It causes maturing of skin.
- Damage skin cells and causes skin malignancies.
- In human eye cornea ingestion of UV – B radiation and high dosage of UV – B causes aggravation of cornea called snow-blindness, cataract and so on.
- Such exposures may harm cornea.
Prevention
- Montreal Protocol was marked at Montreal (Canada) in 1987 to control outflow of ozone draining substances
- Many endeavors are being made to lessen emanation of ozone draining substances
Degradation by Improper Resource Utilization and Maintenance
Soil Erosion
The expulsion of top rich layer because of human exercises
Reasons:
- Over cultivation
- Deforestation
- Unrestricted grazing
- Poor irrigation practices
Water logging and soil salinity
- Irrigation within appropriate waste disposal prompts to water holding up in the soil
- Brings up the salt to the surface of the soil
- The salt begins gathering at the roots of the plants
- The salt harms the roots and yield creations
Deforestation
Conversion of forested regions to non-forested one.
- How deforestation does occurs:
- Slash and blaze farming/jhum development
- Farmers chop down the trees of the timberland and smolder the plant remains
- Ash is utilized as manure and land is utilized for cultivating or steers grazing
- Later, Land is left uncultivated for quite a while for renewal of minerals
Effects of deforestation
- Leads to global warming due to excess carbon-dioxide
- Damage to hydrological cycle
- Loss of biodiversity
- Desertification of land
- Leads to soil erosion
Reforestation
- Restoring timberland that was existing before that is. Celebrating Van-Mahotsavas.
- It additionally happens naturally
- Afforestation: Developing a timberland in another range where no such backwoods existed around there.

Fig: Protect Earth
A case study of people‘s participation in forest conservation
- A lord of Jodhpur needed to organize wood for his new castle in 1731.
- Few Bishnois embraced the trees and requested that cut them first instead of cutting trees.
- 365 people lost their lives in this demonstration
- A small temple is currently present there in recognition of this demonstration
- Amrita Devi Bishnois Wild Life Protection Award is established for people of provincial territories who appreciate ensuring natural life.
Chipko Movement
- It was begun by neighborhood ladies of Garhwali; they embraced the trees to shield them from the tomahawks of temporary workers.
- Joint forest management (JFM)
- Strategy Government of India in 1980
- Local people group worked with the legislature to spare the woodland.
- Communities get woodland items for consolation.
CPCB: Central Pollution Control Board
BOD: Biological Oxygen Demand
CNG: Compressed Natural Gas
FOAM: Friends of Arcata Marsh
JFM: Joint Forest Managemen