- IB Style Question Banks with Solution
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 1
- IB DP Biology SL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
- IB DP Biology HL- IB Style Practice Questions with Answer-Topic Wise-Paper 2
8.1 Metabolism
Essential Idea:
Metabolic reactions are regulated in response to the cell’s needs
Understandings:
- Metabolic pathways consist of chains and cycles of enzyme-catalysed reactions
- Enzymes lower the activation energy of the chemical reactions that they catalyse
- Enzyme inhibitors can be competitive or non-competitive
- Metabolic pathways can be controlled by end-product inhibition
Applications:
- End-product inhibition of the pathway that converts threonine to isoleucine
- Use of databases to identify potential new anti-malarial drugs
Skills:
- Calculating and plotting rates of reaction from raw experimental results
- Distinguishing different types of inhibition from graphs at specified substrate concentration
- Contrast metabolic chain reaction pathways with cyclical reaction pathways.
- Define activation energy.
- Explain the role of enzymes in lowering the activation energy of a reaction.
- Define enzyme inhibitor.
- Contrast competitive and noncompetitive enzyme inhibition.
- Outline one example of a competitive enzyme inhibitor and one example of a noncompetitive enzyme inhibitor.
- Describe allosteric regulation of enzyme activity.
- Outline the mechanism and benefit of end-product inhibition.
- Illustrate end-product inhibition of the threonine to isoleucine metabolic pathway.
- State the consequence of an increase in isoleucine concentration.
- Outline the reasons for development of new anti-malarial drugs.
- Explain the use of databases in identification of potential new anti-malarial drugs.
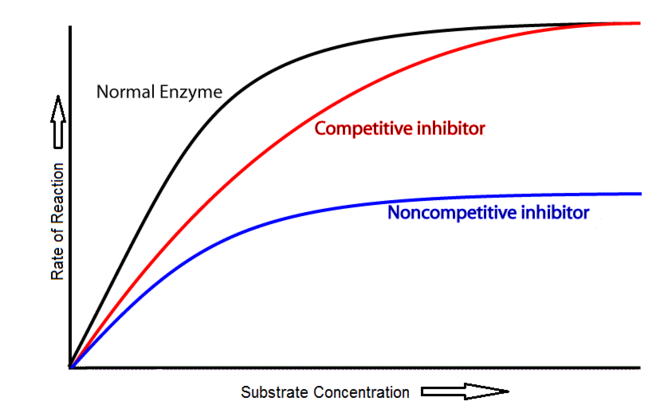
- Explain why the rate of reaction with increasing substrate concentration is lower with a non-competitive inhibitor compared to a competitive inhibitor.
- State two methods for determining the rate of enzyme controlled reactions.
- State the unit for enzyme reaction rate.
- Given data, calculate and graph the rate of an enzyme catalyzed reaction.
- Outline the use and benefits of the bioinformatics technique of chemogenomics in development of new pharmaceutical drugs.
Topic 8.1: Metabolism

In the Metabolism Unit we will learn the importance of enzymes and how they speed up metabolic pathways. We will also look at enzyme inhibitors and how metabolic pathways can be regulated through feedback mechanisms.
This unit will last 4 school days
Essential idea:
- Metabolic reactions are regulated in response to the cell’s needs.
Nature of science:
- Developments in scientific research follow improvements in computing—developments in bioinformatics, such as the interrogation of databases, have facilitated research into metabolic pathways. (3.8)
- Outline the use and benefits of the bioinformatics technique of chemogenomics in development of new pharmaceutical drugs.
8.1 U 1 Metabolic pathways consist of chains and cycles of enzyme-catalysed reactions.
- Contrast metabolic chain reaction pathways with cyclical reaction pathways.
Some of these pathways are anabolic, which is building up of organic molecules (easy to remember as anabolic steroids help build muscle). The other pathways are catabolic, which means breaking down of large organic molecules into smaller ones (example – hydrolysis reactions during digestion)
Some of these metabolic reactions are cycles (i.e. Krebs Cycle) and some are linear chains (i.e. Glycolysis)
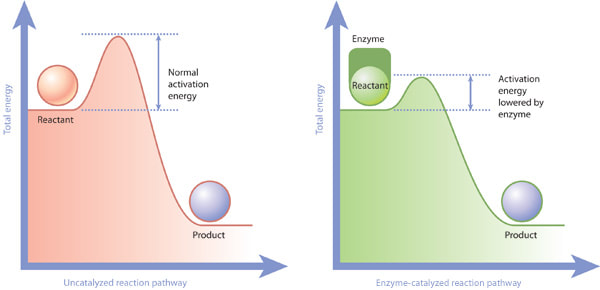
8.1 U 2 Enzymes lower the activation energy of the chemical reactions that they catalyse. (Oxford Biology Course Companion page 374)
- Define activation energy.
- Explain the role of enzymes in lowering the activation energy of a reaction.
Activation energy more specifically can be defined as the energy needed to weaken and break the chemical bonds of the substrate. Enzymes work by lowering the activation energy needed for the reaction to occur.
Chemical reaction
- reactants converted into products
Activation energy
- must be exceeded for any reaction to occur
- allows breaking of bonds in exergonic reactions
- allows formation of new bonds in endergonic reactions
Enzymes
- reduce activation energy
- active site interacts with substrate, altering stability of substrate bonds, allowing substrate molecules to form a transition state which is different than would be formed without the enzyme
- transition state of enzyme-catalyzed reactions has lower energy than non-enzyme-catalyzed reactions, thus lowering activation energy
- the net energy released in exergonic reactions, or taken in by endergonic reactions, is not changed by the enzyme, which only reduces activation energy by lowering the energy requirements of the transition state of reactants
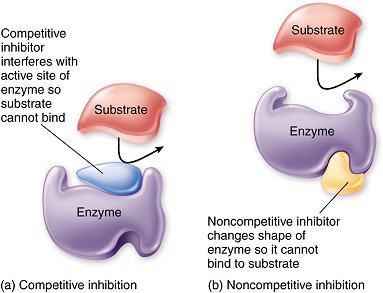
8.1 U 3 Enzyme inhibitors can be competitive or non-competitive. (Guidance: enzyme inhibition should be studied using one specific example for competitive and non-competitive inhibition.) (Oxford Biology Course Companion page 375)
- Define enzyme inhibitor.
- Contrast competitive and noncompetitive enzyme inhibition.
- Outline one example of a competitive enzyme inhibitor and one example of a noncompetitive enzyme inhibitor.
Competitive inhibition occurs when a molecule that is structurally similar to the substrate competes directly with substrate for access to the active site, thus decreasing the number of times a substrate interacts with an enzyme. Non-competitive inhibition occurs when an inhibitor does not compete for the active site with the substrate, but instead binds to a separate site on the enzyme.
Competitive inhibition
- Substrate and inhibitor are chemically very similar
- Inhibitor binds to the active site of the enzyme
- While the inhibitor occupies the active site, it prevents the substrate from binding, and so the activity of the enzyme is prevented until the inhibitor dissociates. The chemical reaction rate decreases.
- Competitive inhibition is usually reversible but can be irreversible in some cases.
- Competitive inhibition can be overcome by sufficiently increasing the concentrations of substrate, thereby out-competing the inhibitor.
example: folic acid synthetase
- folic acid synthetase is an enzyme in bacteria which normally produces folic acid, an essential vitamin, from PABA and other substrates
- a group of antibiotics, known as sulfanilamides, binds to and occupies the active site of folic acid synthetase, thus blocking the access of the similarly shaped substrate, PABA
- without folic acid, the bacteria die, and the infection is overcome
Non-competitive inhibition
- Non-competitive inhibition is also called allosteric inhibition and the site where the inhibitor binds is called the allosteric site.
- Substrate and inhibitor are not similar
- Inhibitor binds to the enzyme at a site different than the active site
- The inhibitor changes the conformation (3-D, tertiary structure) of the enzyme, causing enough alteration to slow enzyme activity; while substrate binds to the active site, it is not converted to a product
- Non-competitive inhibition is usually reversible.
- Since the inhibitor binds to a site other than the active site, increasing the concentration of the substrate will not speed up the reaction or reduce the effect of the inhibitor
Example: silver, Ag+
- Silver forms bonds with the -SH groups of cysteine, the amino acid which normally forms covalent disulfide bridges
- The disruption of disulfide bridges alters the tertiary structure of the enzyme, affecting its active site
- Silver (and other heavy metals) act as metabolic poisons by disrupting the activity of many enzymes
8.1.U 4 Metabolic pathways can be controlled by end-product inhibition.
- Describe allosteric regulation of enzyme activity.
- Outline the mechanism and benefit of end-product inhibition
Allosteric or non-competitive inhibition plays an important role in controlling metabolic pathways.. When a metabolic pathway is producing a specific product the product will also act as an inhibitor to this metabolic pathway.
- Used to regulate metabolic pathways, either switching them on when needed, or switching them off when not needed
- Binds end products of a metabolic pathway to an allosteric site (away from the active site) the shape of allosteric enzymes can be altered to decrease the activity of these enzymes
- Generally when the end product of a metabolic pathway is formed in excess, the excess products interact with enzymes at the beginning of the pathway, decreasing the activity of the pathway until such a time that the end products are used up, thus releasing the allosteric enzymes from inhibition, and allowing for the metabolic pathway to function again, producing more end products
- This is a negative feedback know as end-product inhibition
Example: threonine deaminase
- Amino acids can be converted into other forms by a series of enzymes, including threonine deaminase, which converts threonine to isoleucine
- When isoleucine is in excess, isoleucine binds to an allosteric site of threonine deaminase, decreasing its activity, and thus inhibiting the production of isoleucine
- The binding of isoleucine to the allosteric site is reversible. Later, when isoleucine is in shortage, it is released from the allosteric site, and threonine deaminase is once again released from inhibition, allowing threonine deaminase to proceed, producing more isoleucine
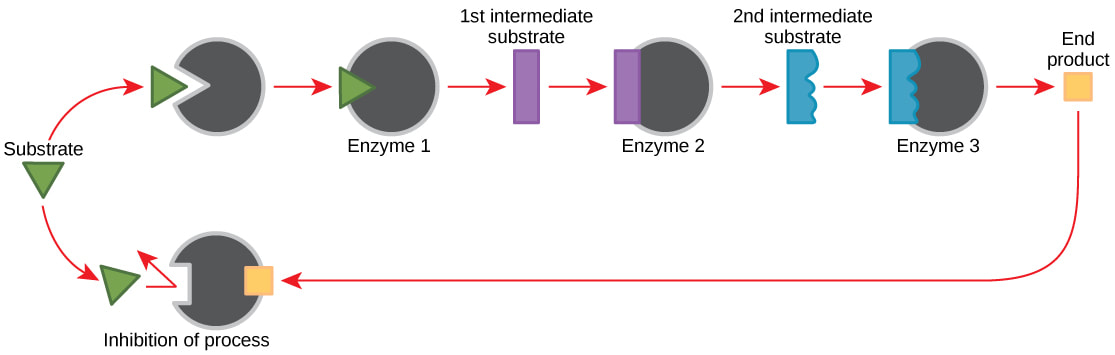
8.1 A 1 End-product inhibition of the pathway that converts threonine to isoleucine.
- Illustrate end-product inhibition of the threonine to isoleucine metabolic pathway.
- State the consequence of an increase in isoleucine concentration.
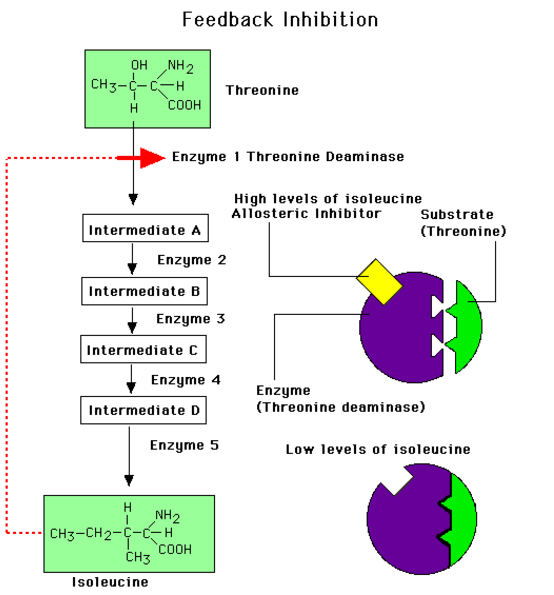
8.1 A 2 Use of databases to identify potential new anti-malarial drugs. (Oxford Biology Course Companion page 378)
- Outline the reasons for development of new anti-malarial drugs.
- Explain the use of databases in identification of potential new anti-malarial drugs.
Importance of databases
- Requires a large investment to develop, license and deploy a new antimalarial drug
- Durg resistance of target populations can quickly devalue the investment
- Important to understand mechanisms of selection, patterns of drug use in different environments and patterns of resistance.
- Databases containing information on the levels of resistance to antimalarial drugs, e.g. publications of P. falciparum genome has made it possible to trace the evolution of highly drug-resistant parasites
- Databases allow researchers to establish and maintain close monitoring of new drugs to malaria useful therapeutic live. Also important to make the database accessible to all
- Malaria is a disease caused by the protist Plasmodium falciparum
- The increased resistance of the pathogen P. falciparum to anti-malarial drugs such as chloroquine and the increasing global efforts to eradicate malaria have driven the need to produce new anti-malarial drugs
P. falciparum strain 3D7
P. falciparum strain 3D7 has been sequenced by scientists and is used to test chemicals for new possible medication.
One specific study tested over 300,000 chemicals against a chloroquine-sensitive 3D7 strain and a chloroquine-resistant K1 strain to determine if any of these chemicals inhibited metabolism. The results showed that 19 new chemicals inhibited the enzymes normally targeted by anti-malarial drugs and 15 chemicals that bound to a total of 61 different malarial proteins. This research provides starting points to produce possible new anti-malarial drugs.
8.1 S 1 Calculating and plotting rates of reaction from raw experimental results. (Oxford Biology Course Companion page 376)
- Explain why the rate of reaction with increasing substrate concentration is lower with a non-competitive inhibitor compared to a competitive inhibitor.
Catalase is an enzyme found in the cells of many tissues of living organisms. It speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances–water and oxygen.
The chemical reaction is as follows: 2H2O2 –> 2H2O + O2
This reaction is important to cells because hydrogen peroxide (H2O2) is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells. The following data has been recorded from the breakdown of hydrogen peroxide by beef liver over a 10 minute time period.
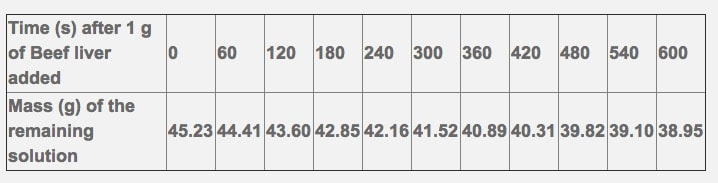
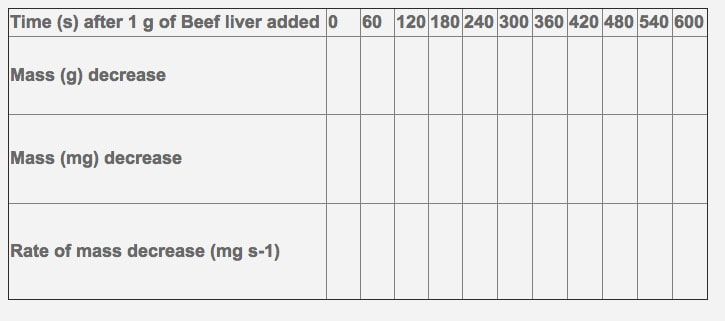
- Please show one sample calculation for the rate of mass decrease over time.
- Please graph your change in mass over your change in time to show the rate (write in the proper labels on the axis, including units.
- Calculate the overall rate of reaction for the 600 second time period.
- What do you notice as the time increases?
- Why do you think this is the case?
8.1 S 2 Distinguishing different types of inhibition from graphs at specified substrate concentration. (Oxford Biology Course Companion page 378)
- State two methods for determining the rate of enzyme controlled reactions.
- State the unit for enzyme reaction rate.
- Given data, calculate and graph the rate of an enzyme catalyzed reaction.
Many enzyme inhibitors have been used in medicine.
For example ethanol has been used to act as a competitive inhibitor for antifreeze poisoning.
Fomepizole, which is an inhibitor of alcohol dehydrogenase, has also been used for antifreeze poisoning.
The main ingredient in antifreeze is called ethylene glycol
- Ethylene glycol is a toxic, colourless and odorless liquid with a sweet taste that is sometimes consumed by children and animals
- Symptoms include feelings of intoxication and nausea, followed by vomiting, hyperventilation, acidosis and acute kidney failure.
- Treatment must start as soon as possible to prevent kidney failure, which can be fatal
Competitive Inhibition
- Ethanol is used as treatment to block the enzyme responsible for metabolizing ethylene glycol into glycolic acid and oxalic acid (these two molecules are more toxic that the original molecule)
- Ethanol acts by competing with ethylene glycol for the active site of alcohol dehydrogenase, the first enzyme in the degradation pathway. Because ethanol has a much higher affinity for the alcohol dehydrogenase, about a 100-times greater affinity, it successfully blocks the breakdown of ethylene glycol into glycoaldehyde, which prevents the further degradation into its dangerous metabolites such as oxalic acid.
- Since the oxalic acid isn’t formed, kidney damage is avoided and the ethylene glycol is excreted in the urine
Non-Competitive Inhibition
- Fomepizole is a strong inhibitor that also blocks the formation of the destructive metabolites of ethylene glycol and the approved antidote by the US Food and drug Administration
- Fomepizole binds to the allosteric site of the enzyme which changes the conformational shape of the enzyme
- The ethylene glycol can no longer bind to the active site
- Since this is non-competitive dosage adjustments and blood monitoring do not have to be performed as with ethanol treatment (ethanol treatments require frequent blood ethanol measurements and dosage adjustments to maintain therapeutic ethanol concentrations as the ethanol is broken down)
- A disadvantage is that Fomepizole is expensive