IB PHYSICS SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 3.1 – Thermal Concepts
Topic 3 Weightage : 6 %
All Questions for Topic 3.1 – Molecular theory of solids, liquids and gases , Temperature and absolute temperature , Internal energy , Specific heat capacity , Phase change , Specific latent heat
Question
When 40 kJ of energy is transferred to a quantity of a liquid substance, its temperature increases by 20 K. When 600 kJ of energy is transferred to the same quantity of the liquid at its boiling temperature, it vaporizes completely at constant temperature. What is

for this substance?
A. 15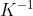
B. 15 K
C. 300 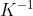
D. 300 K
▶️Answer/Explanation
Ans: D
Ref: https://www.iitianacademy.com/ib-physics-unit-3-thermal-concepts-notes/
SPECIFIC HEAT CAPACITY
\(600 \;kJ = m \times L\)
\(\therefore \frac{m \times L}{m\times c\times \Delta T} =\frac{600 \;kJ}{40 \;kJ}\)
or
\(\frac{L}{c} =\frac{600 \;kJ}{40 \;kJ}\times \Delta T =300 K\)
Question
Which aspect of thermal physics is best explained by the molecular kinetic model?
A The equation of state of ideal gases
B The difference between Celsius and Kelvin temperature
C The value of the Avogadro constant
D The existence of gaseous isotopes
▶️Answer/Explanation
Ans: A
Ref:https://www.iitianacademy.com/ib-physics-unit-3-modelling-a-gas-notes/
The kinetic molecular theory can be used to explain each of the experimentally determined gas laws.
Any sample of a gas is made of molecules. A molecule is the smallest unit having all the chemical properties of the sample. The observed behaviour of a
gas results from the detailed behaviour of its large number of molecules. The kinetic theory of gases attempts to develop a model of the molecular behaviour
which should result in the observed behaviour of an ideal gas.
Question
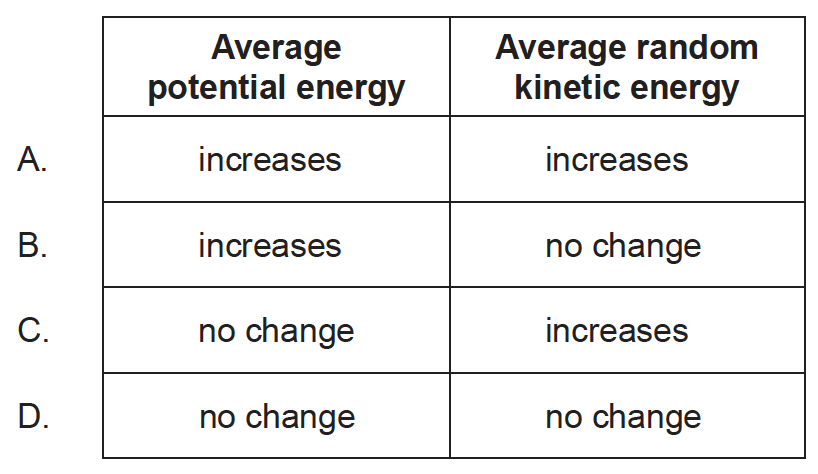
Molecules leave a boiling liquid to form a vapour. The vapour and the liquid have the same temperature.
What is the change of the average potential energy and the change of the average random kinetic energy of these molecules when they move from the liquid to the vapour?
▶️Answer/Explanation
Markscheme
B
During a phase change, temperature of the substance remains the same. Average kinetic energy of the molecules of the substance does not change during phase change but average potential energy changes.
Heat supplied at phase change is used in increasing internal energy of molecules. Internal energy of a given body in vapour phase is larger than that in liquid phase
Question
Which of the following is equivalent to a temperature of –100°C?
A. –373 K
B. –173 K
C. 173 K
D. 373 K
▶️Answer/Explanation
Markscheme
C
TºK = (tºC + 273) or tºC = (TK – 273)
Hence –100°C in K is –100+273 = 173K
Question
A sample of solid copper is heated beyond its melting point. The graph shows the variation of temperature with time.
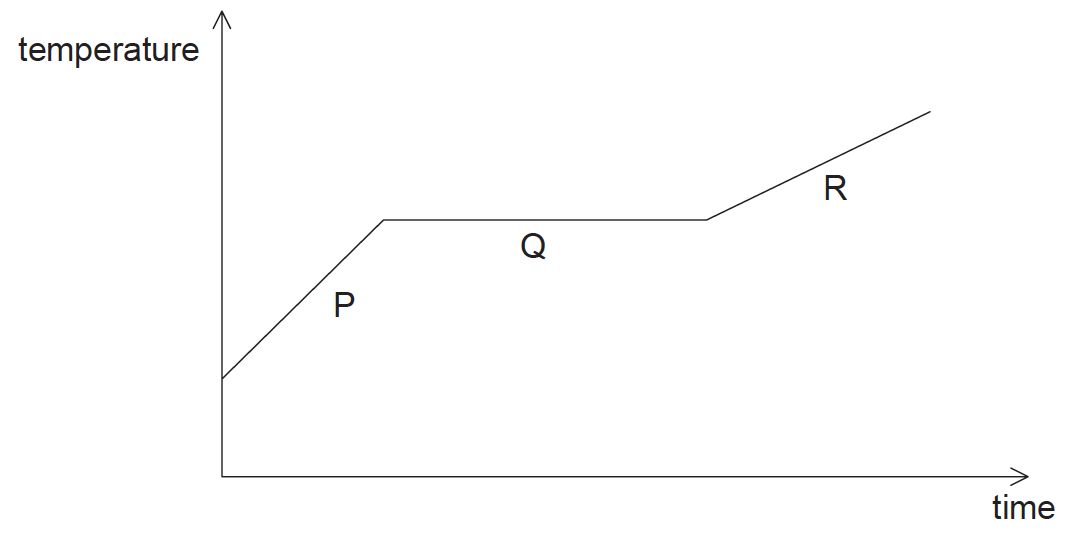
During which stage(s) is/are there an increase in the internal energy of the copper?
A. P, Q and R
B. Q only
C. P and R only
D. Q and R only
▶️Answer/Explanation
Markscheme
A
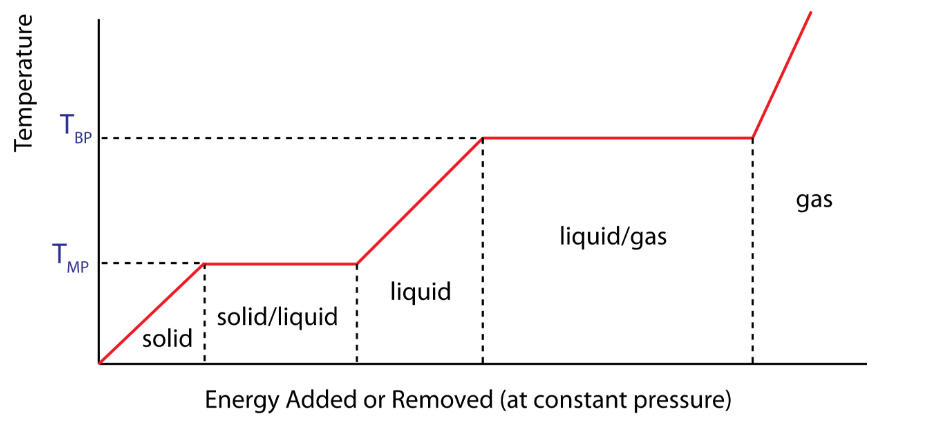
Question
A pure solid is heated at its melting point. While it is melting the
A. mean kinetic energy of the molecules of the solid increases.
B. mean potential energy of the molecules of the solid increases.
C. temperature of the solid increases.
D. temperature of the solid decreases.
▶️Answer/Explanation
Markscheme
B

during Phase change (melting) there is no change in Temperature and hence Kinetic Energy remain constant. heat supplied \(Q=mL\) is used to increase internal potential energy.
Question
Equal masses of water at 80°C and paraffin at 20°C are mixed in a container of negligible thermal capacity. The specific heat capacity of water is twice that of paraffin. What is the final temperature of the mixture?
A. 30°C
B. 40°C
C. 50°C
D. 60°C
▶️Answer/Explanation
Markscheme
D
Let the Final Temperature of mixture be t0 C
Loss of heat by Water = Gain of Heat by paraffin
\(ms_w(80-t)=ms_p(t-20)\)
\((80-t)\times 2s_p=s_p(t-20)\)
\(160-2t=t-20\)
\(t=60^{\circ}C\)
