Question
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
(i) Deduce two features of this molecule that can be obtained from the mass spectrum. Use the data booklet. [2]
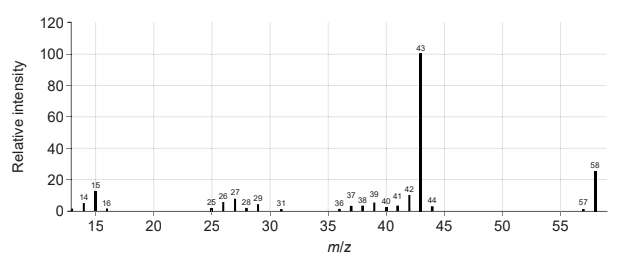
m/z 58:
m/z 43:
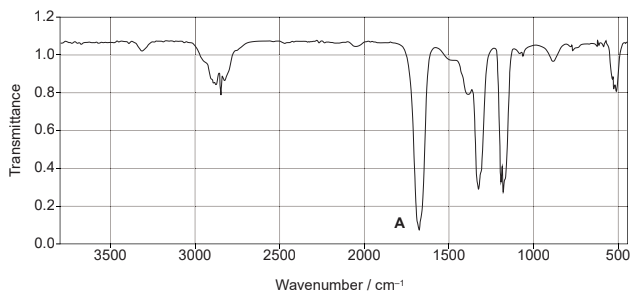
(ii) Identify the bond responsible for the absorption at A in the infrared spectrum. Use the data booklet. [1]
(iii) Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet. [2]
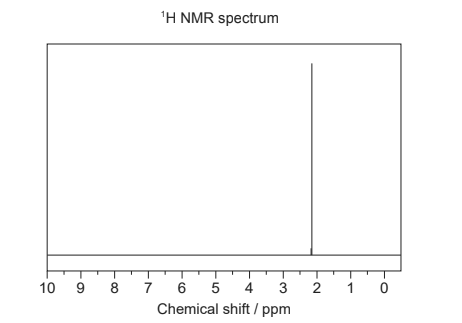
Information deduced from 1H NMR:
Compound:
▶️Answer/Explanation
Ans
i
m/z 58:
molar/«relative» molecular mass/weight/Mr «is 58 g mol−1 /58» ✓
m/z 43:
«loses» methyl/CH3 «fragment»
OR COCH3 + «fragment» ✓
Do not penalize missing charge on the fragments.
Accept molecular ion «peak»/ CH3COCH3 + /C3H6O + . Accept any C2H3O + fragment/ CH3CH2CH2 + /C3H7 +
ii
C=O
Accept “carbonyl/C=C”.
iii
Information deduced from 1H NMR:
«one signal indicates» one hydrogen environment/symmetrical structure
OR «chemical shift of 2.2 indicates» H on C next to carbonyl Compound: propanone/CH3COCH3
Question
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
\(\begin{array}{*{20}{l}} {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{{\text{(OH)}}}_{\text{2}}}{\text{(aq)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{177.0 kJ}}} \\ {{\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}}&{\Delta {H^\theta } = + {\text{189.2 kJ}}} \\ {{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{{\text{1}}}{{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{285.5 kJ}}} \end{array}\)
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJ\(\,\)kg−1\(\,\)K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
Identify the species responsible for the peak at m/z = 110 in the mass spectrum of hydroquinone.
Identify the highest m/z value in the mass spectrum of quinone.
▶️Answer/Explanation
Markscheme
ΔH = 177.0 – \(\frac{{189.2}}{2}\) –285.5 «kJ»
«ΔH =» –203.1 «kJ»
Accept other methods for correct manipulation of the three equations.
Award [2] for correct final answer.
[2 marks]
203.1 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 57.2 «K»
«Tfinal = (57.2 + 21.8) °C =» 79.0 «°C» / 352.0 «K»If 200.0 kJ was used:
200.0 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 56.3 «K»
«Tfinal = (56.3 + 21.8) °C =» 78.1 «°C» / 351.1 «K»
Award [2] for correct final answer.
Units, if specified, must be consistent with the value stated.
[2 marks]
C6H4(OH)2+
Accept “molecular ion”.
Do not accept “C6H4(OH)2” (positive charge missing).
[1 mark]
«highest m/z» 108
Only accept exactly 108, not values close to this.
[1 mark]
