Question
Amino acids are the building blocks of proteins.
a. Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
b. Deduce the number of ${ }^1 \mathrm{H}$ NMR signals produced by the zwitterion form of alanine.
c. Outline why amino acids have high melting points.
▶️Answer/Explanation
Markscheme
a.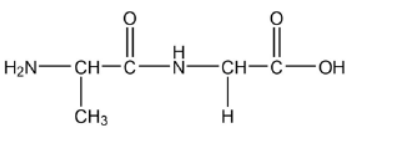
peptide bond
order of amino acids
Accept zwitterion form of dipeptide.
Accept a condensed structural formula or a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
[2 marks]
b. 3
[1 mark]
c. form zwitterions
«strong» ionic bonding
OR
«strong» ionic lattice
OR
«strong» electrostatic attraction/forces
Do not accept hydrogen bonding or IMFs for M2.
[2 mark]
Question
Polymers have a wide variety of uses but their disposal can be problematic.
a. Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
b. The infrared (IR) spectrum of polyethene is given.
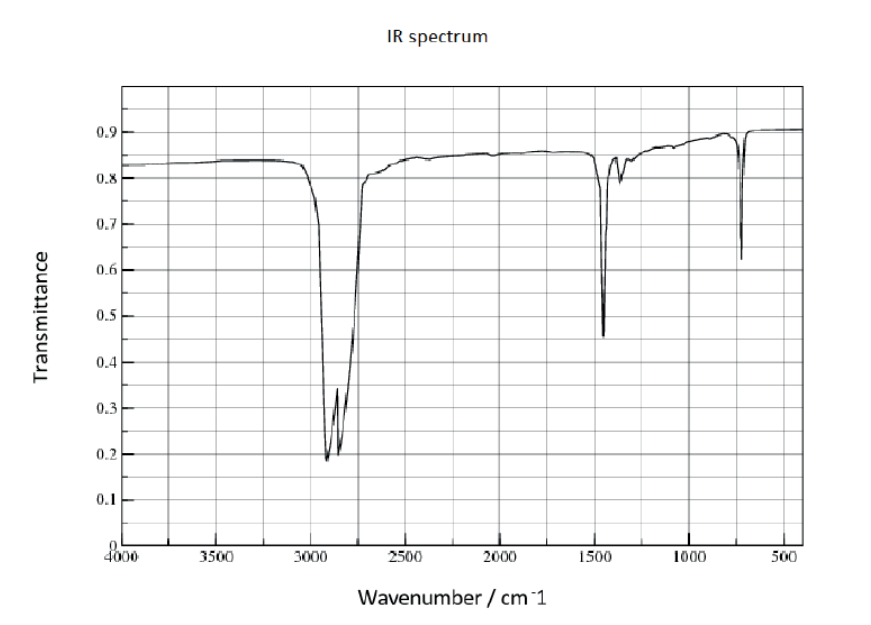
Suggest how the IR spectrum of polychloroethene would differ, using section 26 of the data booklet.
c. Identify a hazardous product of the incineration of polychloroethene.
d. Explain how plasticizers affect the properties of plastics.
e. Suggest why the addition of plasticizers is controversial.
▶️Answer/Explanation
Markscheme
a.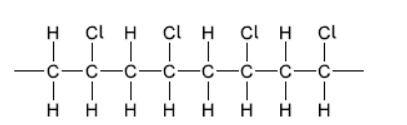
correct bonding $[\boldsymbol{U}]$
$\mathrm{Cl}$ atoms all on same side and alternate $[\boldsymbol{V}]$
Note: Continuation bonds must be shown.
Award [1 max] if less than or more than four units shown.
Accept a stereo formula with all atoms and bonds shown.
b. «strong additional» absorption at $600-800 \ll \mathrm{cm}^{-1} »[\boldsymbol{\sim}]$
c. Any one of:
$\mathrm{HCl}[\boldsymbol{\sim}]$
$\mathrm{Cl}_2[\boldsymbol{\sim}]$
dioxins $[\boldsymbol{\sim}]$
$\mathrm{C}[\boldsymbol{\varkappa}]$
$\mathrm{CO}[\boldsymbol{\nu}]$
d. Any two of:
embedded/fit between chains of polymers [ $\boldsymbol{U}$ ]
keep polymer strands/chains/molecules separated/apart [ $\boldsymbol{\swarrow}]$
increase space/volume between chains $[\boldsymbol{\swarrow}]$
weaken intermolecular/dipole-dipole/London/dispersion/instantaneous dipoleinduced dipole/van der Waals/vdW forces «between chains» [ $\boldsymbol{C}]$
increase flexibility/durability/softness $[\boldsymbol{\sim}]$
make polymers less brittle $[\boldsymbol{\sim}]$
e. leach into foodstuffs/environment
OR
«unknown» health/environmental consequences [ $/$ ]
Note: Accept “plasticizers cannot be recycled”.
Question
Octane number is a measure of the performance of engine fuel.
a. Suggest why a high-octane number fuel is preferable.
b(i)Reforming reactions are used to increase the octane number of a hydrocarbon fuel.
Suggest the structural formulas of two possible products of the reforming reaction of heptane, $\mathrm{C}_7 \mathrm{H}_{16}$.
$\mathrm{b}$ (ii)The ${ }^1 \mathrm{H}$ NMR spectrum of one of the products has four signals. The integration trace shows a ratio of the areas under the signals of $9: 3: 2: 2$.
Deduce the structural formula of the product.
▶️Answer/Explanation
Markscheme
a. low knocking/auto-ignition
NOTE: Do not accept “pre-ignition”.
OR
more efficient fuel
NOTE: Accept “less $\mathrm{CO}_2$ emissions since knocking engine uses more fuel “to produce the same power»”.
OR
high compression
OR
more power extracted
OR
more air going into engine / turbocharging
OR
less engine damage
b(i)Any two of:
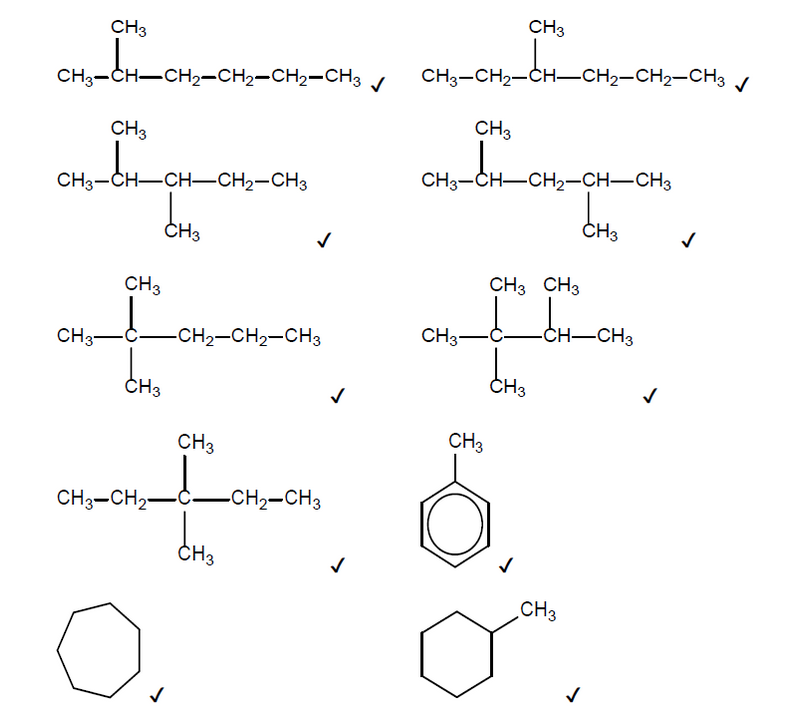
NOTE: Accept skeletal formulas or full or condensed structural formulas.
Accept any other branched cycloalkane that contains 7 carbons.
Do not accept any alkenes.
Penalise missing hydrogens or bond connectivities once only in Option C.
Accept hydrogen as the second product if the first product is toluene or a cycloalkane.
b(ii).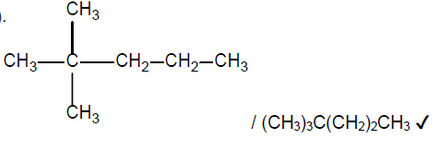
NOTE: Accept a skeletal formula or a full or condensed structural formula. Penalise missing hydrogens or bond connectivities once only in Option C.
Question
Explain what occurs at a molecular level during the absorption of infrared (IR) radiation by the sulfur dioxide molecule, \({\text{S}}{{\text{O}}_{\text{2}}}\).
Consider the IR spectra of the following three compounds.
\[\begin{array}{*{20}{l}} {{\text{A}} = {\text{C}}{{\text{H}}_{\text{3}}}{{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COOH}}} \\ {{\text{B}} = {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}} \\ {{\text{C}} = {{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COH}}} \end{array}\]
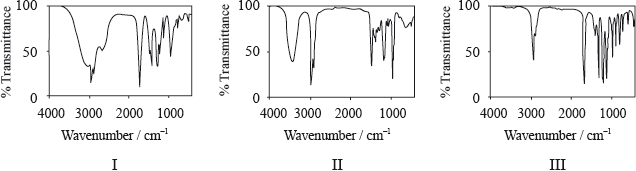
Determine which IR spectrum corresponds to each compound A, B and C. Explain your reasoning. IR data can be found in Table 17 of the Data Booklet.
▶️Answer/Explanation
Markscheme
(O–S–O) bond angle changes;
(S–O) bond (length) stretches;
Allow [1] for S–O bond vibrations if neither of the above points are scored.
A is Spectrum I and B is Spectrum III and C is Spectrum II;
A Spectrum I:
only spectrum with a (broad) peak in the range \({\text{2500–3300 (c}}{{\text{m}}^{ – 1}}{\text{)}}\) corresponding to the carboxylic acid functional group / –OH in carboxylic acid / H-bonding in carboxylic acid (so must be a carboxylic acid);
B Spectrum III:
peak in the range \({\text{1700–1750 (c}}{{\text{m}}^{ – 1}}{\text{)}}\) corresponding to the carbonyl/C=O group;
but no peak for O–H/no peak at \({\text{2500–3300 (c}}{{\text{m}}^{ – 1}}{\text{)}}\) or \({\text{3200–3600 (c}}{{\text{m}}^{ – 1}}{\text{)}}\);
C Spectrum II:
peak in the range \({\text{3200–3600 (c}}{{\text{m}}^{ – 1}}{\text{)}}\) corresponding to the alcohol functional group/OH / the only one without a peak at \({\text{1700–1750 (c}}{{\text{m}}^{ – 1}}{\text{)}}\) corresponding to a carbonyl/C=O group;
Examiners report
For part (b) candidates often missed discussing the change of dipole moment.
Part (d) illustrated candidates’ ability at linking wave numbers from IR spectra to correct bonds but they did not always provide adequate explanations for their choices.
Question
Distinguish between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of 1-bromopropane and 2-bromopropane (splitting patterns are not required).
▶️Answer/Explanation
Markscheme
2-bromopropane will show two separate absorptions/peaks;
in the ratio 6:1;
1-bromopropane will show three separate absorptions/peaks;
in the ratio 3:2:2;
Examiners report
In (a) most candidates correctly determined the difference in the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of 1- bromopropane and 2 – bromopropane but neglected to mention or misinterpreted the differences in area under the peaks.
Question
Mass spectrometry is a powerful analytical technique used in the identification of organic compounds. The mass spectrum of a compound with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\) displays peaks at m/z 15, 45 and 60.
Determine the molecular formula of the compound.
Identify the fragments responsible for the peaks at
m/z = 15
m/z = 45
Identify a compound that could produce this spectrum.
▶️Answer/Explanation
Markscheme
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}\);
No mark for (CH2O)2.
m/z = 15
\({\text{CH}}_3^ + \);
m/z = 45
\({\text{COO}}{{\text{H}}^ + }{\text{/C}}{{\text{O}}_{\text{2}}}{{\text{H}}^ + }{\text{/HCO}}{{\text{O}}^ + }{\text{/OCO}}{{\text{H}}^ + }\);
Penalize once if charges are missing.
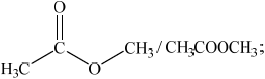
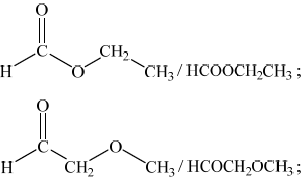
ethanoic acid/ \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) / methyl methanoate/ \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\);
Accept acetic acid.
Examiners report
Most candidates gave the correct molecular formula (a) and many candidates identified the fragments correctly.
Many candidates did not give the charge of the ion.
Many candidates identified acetic acid.
Question
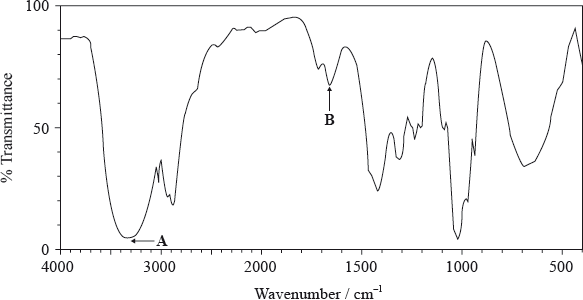
Infrared spectroscopy is an analytical technique that uses electromagnetic radiation.
The infrared spectrum of a substance, X, with empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) is given below.
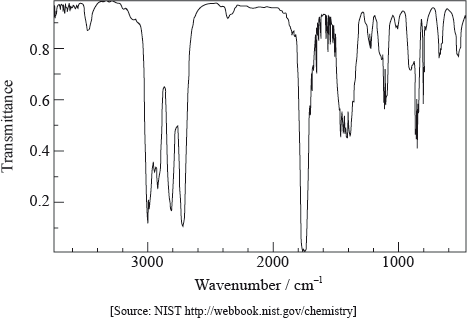
Explain why the structural formula of X cannot be:
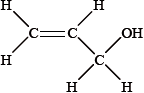
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X consists of three peaks. Deduce the structural formula of X and the relative areas under each peak.
▶️Answer/Explanation
Markscheme
absence of peak between 3200–3600 \({\text{c}}{{\text{m}}^{ – 1}}\)/above 3000 \({\text{c}}{{\text{m}}^{ – 1}}\)/peak for OH;
presence of peak between 1700–1750 \({\text{c}}{{\text{m}}^{ – 1}}\)/peak for C=O;
absence of peak between 1610–1680 \({\text{c}}{{\text{m}}^{ – 1}}\) /peak for C=C;
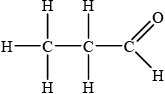 ;
;
Accept CH3CH2CHO.
3:2:1;
Ignore order
ECF if structure is incorrect only if its NMR spectrum contains three peaks.
Examiners report
Many candidates only gave at least one reason why X cannot be the given formula.
Many gave the correct formula for X, but some could not identify the relative areas under the peaks.
Question
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) and IR spectroscopy both involve the absorption of electromagnetic radiation.
Identify the region of the electromagnetic spectrum used in \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy.
Identify which of these two techniques involves higher energy radiation.
▶️Answer/Explanation
Markscheme
radiowaves;
IR / infrared;
Examiners report
Candidates had few problems in (a), but although the correct selection of HCl was usually made in (b), many lost a mark as they did not refer to the change of dipole during the absorption of IR radiation.
Candidates had few problems in (a), but although the correct selection of HCl was usually made in (b), many lost a mark as they did not refer to the change of dipole during the absorption of IR radiation.
Question
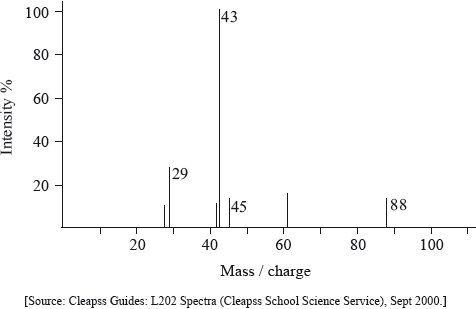
The mass spectrum of an unknown compound, X, of empirical formula C2H4O is shown below.
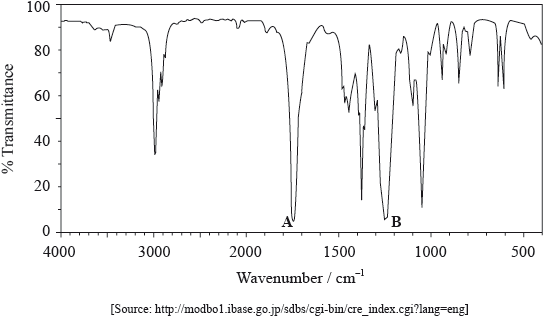
The IR spectrum of X is shown below.
Determine the relative molecular mass of X from the mass spectrum and deduce the formula of the molecular ion.
Identify a fragment which gives rise to the peak at m/z = 29.
Comment on the absence of a peak at m/z = 59.
Use Table 17 of the Data Booklet to identify the bonds which correspond to the absorptions A and B.
A:
B:
Deduce the name of the functional group present in X.
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks. State the information that can be obtained from the number of peaks.
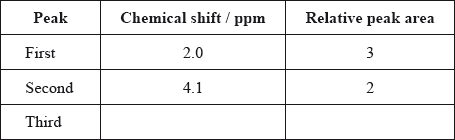
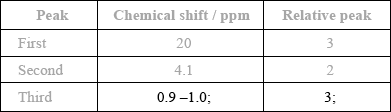
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X includes peaks at 2.0 and 4.1 ppm. Use Table 18 of the Data Booklet to suggest the chemical shift of the third peak and state its relative peak area. Show your answers in the table below.
Deduce a possible structure for X that is consistent with the mass, IR and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra.
▶️Answer/Explanation
Markscheme
88;
Do not award mark if units are given.
\({{\text{C}}_4}{{\text{H}}_8}{\text{O}}_2^ + \);
\({\text{C}}{{\text{H}}_3}{\text{CH}}_2^ + /{{\text{C}}_2}{\text{H}}_5^ + /{\text{CH}}{{\text{O}}^ + }\);
Only penalize once for missing charge in (a) (i) and (ii).
C2H3O2 produced has no charge / fragment produced after loss of C2H5 from molecular ion has no charge;
Accept fragment(s) too unstable, fragment breaks up etc.
Do not accept answers with reference to 13C/14C isotopes and peak at m/z = 61.
Do not accept C2H3O2+ / C3H7O+ does not exist.
A: C=O and B: C–O;
No mark if two bonds are given for A or B.
Ignore names if incorrect.
ester;
Do not accept COO.
the number of different hydrogen/proton environments / OWTTE;
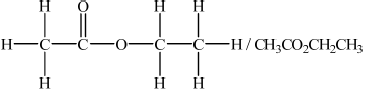 ;
;
Examiners report
In (a), although most identified the formulas of the molecular ion in (i) and the fragment in (ii), the positive charge was often missing.
In (a), although most identified the formulas of the molecular ion in (i) and the fragment in (ii), the positive charge was often missing.
Very few candidates were able to explain the absence of a peak at m/z = 29 in (iii) in the mass spectrum. This is because the fragment produced after loss of C2H5+ has no charge.
A significant number referred to the C–F bond in (b), even though the empirical formula of X given in the question contained no fluorine.
A significant number referred to the C–F bond in (b), even though the empirical formula of X given in the question contained no fluorine.
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).
Question
Details of the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of two of these alcohols are given below.
\[\begin{array}{*{20}{l}} {Spectrum 1}&{} \\ {{\text{Two peaks:}}}&{{\text{One at 1.3 ppm (relative to the TMS reference) with an integration trace}}} \\ {}&{{\text{of nine units, and the other at 2.0 ppm with an integration trace of one unit.}}} \\ {Spectrum 2}&{} \\ {{\text{Four peaks:}}}&{{\text{The first at 0.9 ppm with an integration trace of six units.}}} \\ {}&{{\text{The second at 1.7 ppm with an integration trace of one unit.}}} \\ {}&{{\text{The third at 2.1 ppm with an integration trace of one unit.}}} \\ {}&{{\text{The fourth at 3.4 ppm with an integration trace of two units.}}} \end{array}\]
Consider the proton environments present in each of the alcohol molecules when answering the following questions.
The mass spectrum of one of the alcohols shows peaks at m/z values of 74, 59 and 45.
(i) Identify which alcohol gives spectrum 1 and explain your answer by stating which hydrogen atoms in the molecule are responsible for each of the two peaks.
(ii) Deduce which alcohol gives spectrum 2. Explain which particular hydrogen atoms in the molecule are responsible for the peaks at 0.9 ppm and 3.4 ppm.
(i) Deduce which two of the alcohols could produce this spectrum and identify the species responsible for the three peaks.
(ii) The spectrum also shows a significant peak at m/z = 31. Suggest which alcohol is responsible for this spectrum and deduce the species responsible for the peak at m/z = 31.
Explain why the infrared spectra of all four alcohols are very similar.
▶️Answer/Explanation
Markscheme
(i) (2-)methylpropan-2-ol;
the (H atoms in the three) –CH3 groups are responsible for the peak at 1.3 ppm (as there are 9 H atoms in the same environment with no H on the adjacent C atom);
the –OH hydrogen atom is responsible for the peak at 2.0 ppm;
Accept explanations with suitable diagram.
(ii) (2-)methylpropan-1-ol;
the first peak (at 0.9 ppm) is due to the (H atoms in the) two –CH3 groups
(bonded to the second carbon atom) / (CH3)2CHCH2OH;
the peak at 3.4 ppm is due to the (H atoms in the) –CH2– group /
(CH3)2CHCH2OH;
Accept explanations with suitable diagram.
(i) butan-1-ol and butan-2-ol (as they both contain a \(–{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\) group);
74: \({{\text{M}}^ + }{\text{ / }}{{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}^ + }{\text{ / C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{H}}^ + }\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)CH}}_3^ + \);
59: \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{{\text{O}}^ + }{\text{ / (M}}–{\text{C}}{{\text{H}}_{\text{3}}}{{\text{)}}^ + }{\text{ / C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{H}}^ + }\) and
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)CH}}_3^ + {\text{ / C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH}}{{\text{)}}^ + }\);
45: \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{O}}^ + }{\text{ / (M}}–{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{)}}^ + }{\text{ / C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{H}}^ + }\) and \({\text{CH(OH)CH}}_3^ + \);
Accept explained answers instead of formulas.
(ii) butan-1-ol;
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{H}}^ + }\) / \({{\text{(M}}–{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{)}}^ + }\);
Penalize missing + signs once only in parts (b) (i) and (ii).
they all contain O–H (which will give a broad peak at ca. \({\text{3500 c}}{{\text{m}}^{ – 1}}\));
they all contain C–H (which will absorb at ca. \({\text{3000 c}}{{\text{m}}^{ – 1}}\));
Award [1 max] for same functional groups/bonds.
Examiners report
Most candidates correctly identified the alcohols, but only the better candidates could explain which hydrogen atoms in the molecule were responsible for the peaks. One comment on the G2 forms was surprised by the reference to TMS. However, all \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra have chemical shifts relative to TMS, and no questions were asked about its function.
Many forgot the + charge of the fragments passing through a mass spectrometer and so lost marks.
The similar infrared spectra of the alcohols was often explained in general terms with no reference to the particular bonds present.
Question
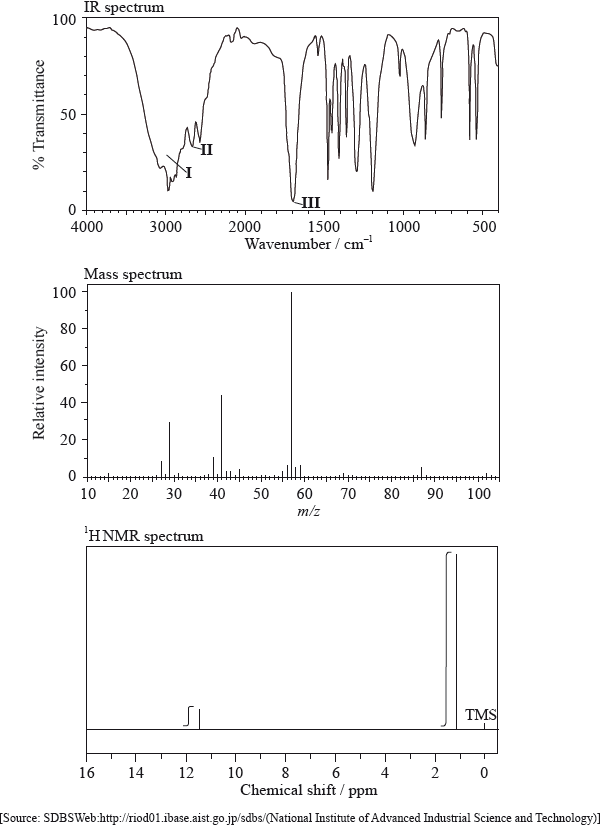
Infrared (IR) spectroscopy is widely used as a technique in analytical chemistry.
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, X, of molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\) are as follows.
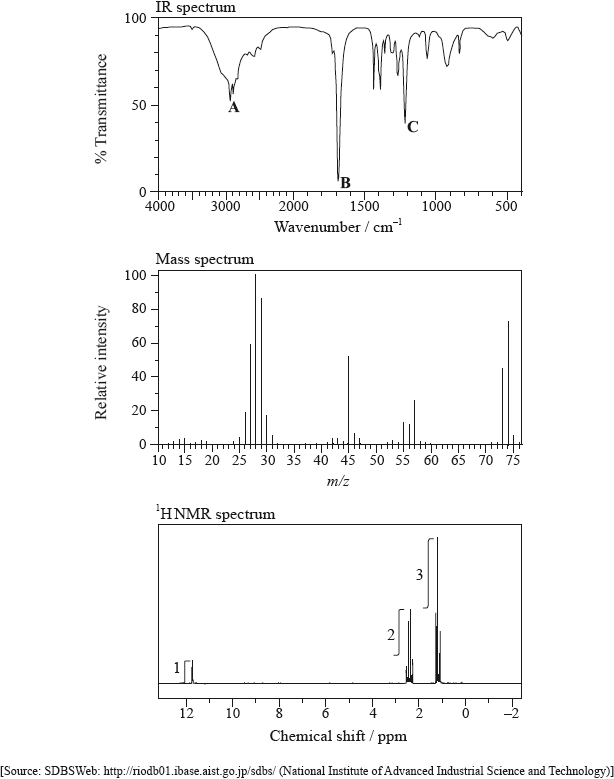
Explain what happens at a molecular level during the absorption of IR radiation by carbon dioxide, CO2.
(i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of X.
A:
B:
C:
(ii) In the mass spectrum of X, deduce which ions the m/z values at 74, 45 and 29 correspond to.
m/z = 74:
m/z = 45:
m/z = 29:
(iii) Identify the peak at 11.73 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
(iv) Deduce the structure of X.
▶️Answer/Explanation
Markscheme
change in bond length / bond stretching / asymmetric stretch;
change in bond angle / bending (of molecule);
Allow [1 max] for only stating vibrations.
induces molecular polarity/dipole moment / OWTTE;
(i) A: O–H
B: C=O
C: C–O
Award [2] for three correct, [1] for two correct.
(ii) m/z = 74: \({{\text{C}}_2}{{\text{H}}_5}{\text{COO}}{{\text{H}}^ + }\) / \({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_2^ + \);
m/z = 45: \({\text{COO}}{{\text{H}}^ + }\);
m/z = 29: \({{\text{C}}_2}{\text{H}}_5^ + \);
Penalize missing + charge once only.
Do not award mark for m/z = 29: CHO+.
(iii) –COOH
Accept –OH.
(iv) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}}\) / \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{O}}_2}{\text{H}}\);
More detailed structural formula may be given.
Examiners report
In (b) the main misconception stated by candidates was that non-polar compounds do not absorb infrared radiation. Most candidates scored a mark for vibrations, but many misunderstood the difference between symmetric and asymmetric stretching.
Part (c)(i) was well answered by the great majority of candidates; giving C–H bond instead of O–H for A was a popular incorrect answer. In (ii) the most common mistake was missing the + sign. Most candidates answered (iii) and (iv) correctly.
Question
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X with molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) is shown below.
The infrared and mass spectra for X were also recorded.
Deduce which of the following compounds is X and explain your answer.
\({\text{C}}{{\text{H}}_{\text{3}}}\)–CO–\({\text{C}}{{\text{H}}_{\text{3}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}\)–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CHO \({\text{C}}{{\text{H}}_{\text{2}}}\)–CH–\({\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound:
Explanation:
Deduce which one of the peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X would also occur in the spectrum of one of the other isomers, giving your reasoning.
Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, that would be present in the infrared spectrum.
Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, absent in this infrared spectrum but present in one of the other compounds shown in part (a).
Suggest the formulas and m/z values of two species that would be detected in the mass spectrum.
Species:
m/z:
Species:
m/z:
▶️Answer/Explanation
Markscheme
Compound:
\({\text{C}}{{\text{H}}_{\text{3}}}\)–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CHO;
Explanation:
only this compound would give 3 peaks / OWTTE;
only this compound has H–atoms in 3 different chemical environments / OWTTE;
only this compound has protons in ratio 3:2:1 in each environment / OWTTE
only this compound would give a peak in the 9.4–10 ppm region / OWTTE;
2.5 ppm peak;
\({\text{C}}{{\text{H}}_{\text{3}}}\)–CO–\({\text{C}}{{\text{H}}_{\text{3}}}\) also has hydrogen atoms on a carbon next to the >C=O group;
1700–1750 \({\text{c}}{{\text{m}}^{ – 1}}\) (>C=O);
1610–1680 \({\text{c}}{{\text{m}}^{ – 1}}\) (>C=C<) / 3200–3600 \({\text{c}}{{\text{m}}^{ – 1}}\) (–O–H);
\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}^ + }\) and m/z = 58;
\({{\text{C}}_{\text{2}}}{\text{H}}_5^ + \) and m/z = 29;
\({\text{CH}}{{\text{O}}^ + }\) and m/z = 29;
\({\text{CH}}_3^ + \) and m/z = 15;
Penalize missing + sign once only.
Examiners report
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Question
Infrared spectroscopy is commonly used as an analytical technique by inorganic, physical and organic chemists.
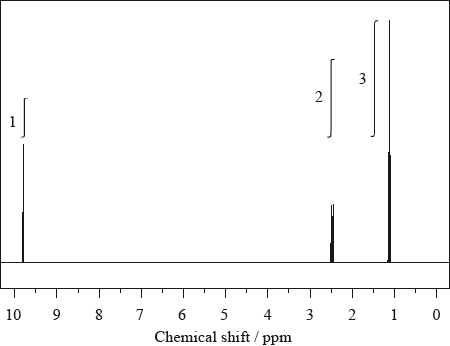
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, X, of molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\), are as follows.
In the IR spectrum, identify the bond responsible for each of the absorptions labelled I, II and III.
I:
II:
III:
In the mass spectrum, deduce which fragments the m/z values at 102, 57 and 45 correspond to.
m/z =102:
m/z = 57:
m/z = 45:
Identify the peak at 11.5 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
State what information can be obtained from the integration traces in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum about the hydrogen atoms responsible for the peak at 1.2 ppm.
Deduce the structure of X.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) is an isomer of X. Deduce two differences between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of this isomer and that of X.
▶️Answer/Explanation
Markscheme
I: O–H;
II: C–H;
III: C=O;
Award [2] for C–H for I and O–H for II.
m/z = 102: molecular ion peak / \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{CCOO}}{{\text{H}}^ + }/{{\text{C}}_5}{{\text{H}}_{10}}{\text{O}}_2^ + /{{\text{M}}^ + }\);
m/z = 57: \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{{\text{C}}^ + }\) / \({{\text{(M}}–{\text{COOH)}}^ + }\) / \({{\text{C}}_4}{\text{H}}_9^ + \);
m/z = 45: \({\text{COO}}{{\text{H}}^{\text{ + }}}\);
Penalize missing + once only.
(H of) COOH group;
nine hydrogens in the same environment / \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{C}}–{\text{(group)}}\);
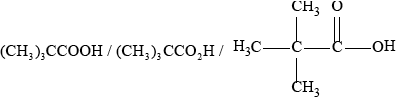 ;
;
no peak at 11.5 ppm in spectrum of isomer / different chemical shift values;
four peaks (instead of two) / different number of peaks;
Three of these peaks can be split in actual spectrum, so allow for this in answers if exactly four peaks is not stated.
different integration trace / different areas under the peaks / integration trace would have a 3:2:2:3 peak area ratio;
Do not award mark if incorrect peak area ratios are given for the structure drawn in (v).
Examiners report
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
In (b) most candidates confused the IR absorptions of O-H and C-H bonds. The positive charge was frequently omitted from the mass spectroscopy fragments and the number of hydrogen atoms in the same chemical environment was often not stated. Most candidates were unable to coordinate all the spectroscopic data to deduce the tertiary structure.
Question
Nuclear magnetic resonance (NMR) and mass spectrometry are diagnostic techniques often used in the identification of organic compounds.
Deduce two similarities and one difference in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of the two isomers \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), a carboxylic acid, and \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\), an ester. \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) data are given in Table 18 of the Data Booklet.
Similarities:
Difference:
The mass spectrum of one of the two isomers above has significant peaks at mass to charge ratios of 15, 45 and 60, while the other isomer has peaks at 15, 29, 31 and 60. Analyse these fragmentation patterns in the two mass spectra in order to distinguish between the two isomers.
▶️Answer/Explanation
Markscheme
Similarities: [2 max]
both have two peaks;
in the same/1:3 ratio;
both have only singlet peaks;
Difference:
\({\text{C}}{{\text{H}}_3}{\text{COOH}}\) will have an absorption/chemical shift/\(\delta \) in the range 2.0–2.5, (\({\text{HCOOC}}{{\text{H}}_3}\) will not) / \({\text{HCOOC}}{{\text{H}}_3}\) will have an absorption in the range 3.8–4.1, (\({\text{C}}{{\text{H}}_3}{\text{COOH}}\) will not) / \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) will have an absorption in the range 9.0–13.0, (\({\text{HCOOC}}{{\text{H}}_3}\) will not);
CH3COOH:
peak at 45 due to \({{\text{(COOH)}}^ + }\) / \({{\text{(}}{M_{\text{r}}} – 15{\text{)}}^ + }\) due to loss of \({\text{C}}{{\text{H}}_3}\);
HCOOCH3:
peak at 31 due to \({{\text{(OC}}{{\text{H}}_3}{\text{)}}^ + }\) / \({{\text{(}}{M_{\text{r}}} – 29{\text{)}}^ + }\) due to loss of HCO/CHO / peak at 29 due to \({{\text{(HCO)}}^ + }\)/\({{\text{(CHO)}}^ + }\) / \({{\text{(}}{M_{\text{r}}} – 31{\text{)}}^ + }\) due to loss of \({\text{OC}}{{\text{H}}_3}\);
Penalize missing + sign once only.
Brackets not required around fragments for marks.
Examiners report
(a) was generally answered well, the commonest errors being to include the 0.9-1.0 ppm chemical shift for the methyl group.
(b) was generally answered well, the commonest errors being omitting the charges on the ions.
Question
A healthy diet consists of a range of food groups in the right proportions that provide the energy for the body to function, grow and repair itself.
Examples of straight-chain fatty acids include \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\), \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) and \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\).
State the empirical formula and structural features of monosaccharides.
Deduce the structural formula of a triester formed from three long-chain carboxylic acid molecules, RCOOH, and one propane-1,2,3-triol molecule, \({\text{HO–C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)–C}}{{\text{H}}_{\text{2}}}{\text{OH}}\). Identify one of the ester linkages in the structure by drawing a rectangle around it.
Deduce the number of C=C bonds present in one molecule of each fatty acid.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\):
▶️Answer/Explanation
Markscheme
\({\text{C}}{{\text{H}}_2}{\text{O}}\);
Accept (CH2O)n
one carbonyl/C=O and (at least two) hydroxyl/OH groups;
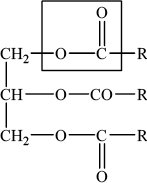
Award [1] for structure that shows unambiguously how the atoms are arranged together.
Award [1] for identifying one of the three ester linkages – must not include R and/or CH2.
\({{\text{C}}_{19}}{{\text{H}}_{39}}{\text{COOH}}\): 0
\({{\text{C}}_{19}}{{\text{H}}_{31}}{\text{COOH}}\): 4
\({{\text{C}}_{19}}{{\text{H}}_{29}}{\text{COOH}}\): 5
All three [2], any two [1], any one [0].
Examiners report
The vast majority of candidates were able to state the empirical formula of monosaccharides, but a good number were not able to state its structural features.
It was surprising to see that only about half could deduce the structure of the triester correctly and identify the ester linkage.
About half of the candidates were able to deduce the number of C=C bonds correctly from the list of fatty acids.
Question
Analytical techniques are very useful in determining molecular structures. A compound, X, has the empirical formula \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{O}}\).
The molecular formula of X is \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). The information in the IR spectrum below can be used to help determine the structure of X.
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks with relative areas of 2:1:1.
Identify the analytical technique that would most readily provide the additional data required to calculate the molecular formula of X.
(i) State what information about a molecule can be obtained from its IR spectrum.
(ii) Deduce the information obtained from absorptions A and B.
A:
B:
(iii) Comment on the absence of any major absorption in the region 1700–1750 \({\text{c}}{{\text{m}}^{ – 1}}\).
(i) Deduce what information can be obtained from these data.
(ii) Deduce the structure of X.
▶️Answer/Explanation
Markscheme
mass spectrometry/spectroscopy / MS;
(i) presence (or absence) of particular bonds;
Accept functional groups.
(ii) A: O–H/hydroxyl;
B: C=C/carbon-carbon double bond;
(iii) no C=O/carbonyl present;
(i) protons/H’s in three different chemical environments / OWTTE;
2:1:1 ratio of protons/H’s (in these environments) / OWTTE;
Accept 4:2:2
(ii) HO–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CH=CH–\({\text{C}}{{\text{H}}_{\text{2}}}\)–OH / \({\text{C}}{{\text{H}}_{\text{2}}}\)=\({\text{C(C}}{{\text{H}}_{\text{2}}}{\text{OH}}{{\text{)}}_{\text{2}}}\);
Examiners report
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.
Question
Deduce the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of 1-bromobutane and 2-bromobutane. Explain how the integration trace can be used to distinguish between the two compounds.
Compare the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 1-bromo-2-methylpropane with the two spectra considered in (a). Include the number of peaks and the integration trace.
▶️Answer/Explanation
Markscheme
both 1-bromobutane and 2-bromobutane have four peaks;
(ratio of areas in) 1-bromobutane: 3:2:2:2;
(ratio of areas in) 2-bromobutane: 3:1:2:3;
For second and third points accept correct ratios given in a different order.
Accept correct number of hydrogen atoms for each peak instead of area.
three peaks (not four);
ratio of areas/integration trace: 6:1:2;
Accept correct ratio given in a different order.
Examiners report
This is a topic that showed better understanding, but many candidates lost marks as their answers did not focus on integration traces. Thus, it looks like quite a few candidates are actually proficient in this technique, but failed to interpret the question properly. Very few candidates could explain the role of \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) in magnetic resonance imaging. Explanations were very shallow evidencing no clear understanding of this technique.
This is a topic that showed better understanding, but many candidates lost marks as their answers did not focus on integration traces. Thus, it looks like quite a few candidates are actually proficient in this technique, but failed to interpret the question properly. Very few candidates could explain the role of \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) in magnetic resonance imaging. Explanations were very shallow evidencing no clear understanding of this technique.
Question
The IR spectrum below represents one of the three organic compounds: propan-1-ol (CH3CH2CH2OH), propanal (CH3CH2CHO) or propanoic acid (CH3CH2COOH).
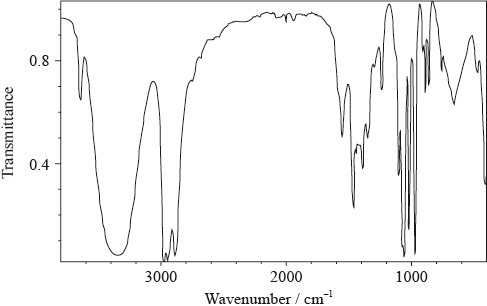
Analyse the spectrum and identify two bonds other than C–H that are present and one that is absent in this compound. Refer to Table 17 of the Data booklet to complete the table.
Bonds present:
Bond absent:
The mass spectrum of the same compound contains strong peaks of \({({M_{\text{r}}} – 15)^ + }\) and \({({M_{\text{r}}} – 17)^ + }\) ions. The first signal corresponds to the loss of a methyl group, \({\text{C}}{{\text{H}}_{\text{3}}}\), from the molecule. Deduce which fragment is lost to produce the second peak.
Using the information above, deduce the identity of the organic compound.
Predict the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of this compound.
▶️Answer/Explanation
Markscheme
Bonds present: [2 max]
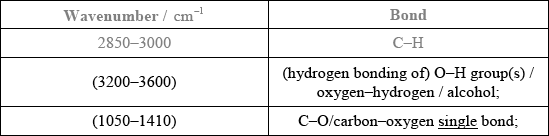
Bonds absent: [1 max]
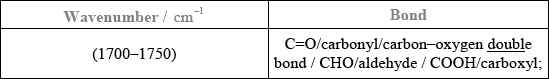
Accept other bonds/groups (C\(\equiv\)C/ alkyne, etc.) even if they do not present in any of the three molecules.
No credit if atoms other than C, H or O are involved.
Award marks for functional group/bond names and ignore wavenumbers.
OH/hydroxyl/alcohol;
Accept +OH.
propan-1-ol/CH3CH2CH2OH;
Accept “alcohol”.
4;
Accept ECF from (c) – if propanoic acid/propanal = 3 peaks.
Examiners report
In (a), the spectrum was well interpreted and few had little difficulty in gaining three marks. The three possible compounds were given in the stem so candidates did not score the last mark if the “absent” bond included an atom not present. One G2 respondent commented that asking students to locate bonds in the fingerprint region is questionable; as it turned out, the candidates had no difficulty with this.
[N/A]
Most were then able to progress through the rest of the question without difficulty although several gave compounds in (c) that were not in the stem of the question.
In (d) the answer given was generally consistent with the answer given in (c).
Question
Magnetic resonance imaging (MRI) is a medical application of NMR spectroscopy.
State one advantage of MRI over X-ray medical imaging with reference to the electromagnetic spectrum.
Outline how MRI is used to scan the human body.
▶️Answer/Explanation
Markscheme
uses no ionizing radiation / uses low-energy radio waves / radio waves safer than x-rays / OWTTE;
Accept “does not damage body tissue”.
MRI is (usually) a proton NMR/\(^{\text{1}}{\text{H}}\,{\text{NMR}}\);
(the states of) protons/hydrogen atoms in water/lipids/carbohydrates/proteins/different (chemical) environments are detected;
different organs have different water concentration;
(strong) magnetic field and radio waves/frequency are used;
(by focusing the scanner on different parts of the body) three-dimensional/3-D
images of (organs in) the body are produced / OWTTE;
Examiners report
Most realized that radio waves are lower in energy than X-rays and so less harmful and in (b) quite a number of possible answers were accepted.
Most realized that radio waves are lower in energy than X-rays and so less harmful and in (b) quite a number of possible answers were accepted.
Question
Compound P contains a carbonyl group (C=O) and has the molecular formula C3H6O.
Pentan-2-one has the following mass spectrum.
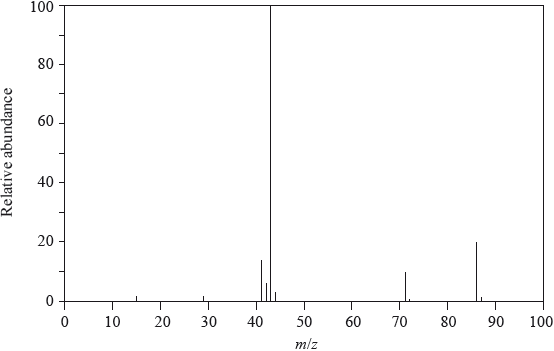
Draw the two possible structures of compound P.
Explain why the infrared spectra of the structures in (a) are very similar.
Explain how the mass spectra of the structures in (a) can be used to distinguish between them.
Deduce the formulas of the species with the m/z values at 86, 71 and 43.
\(m{\text{/}}z = 86\):
\(m{\text{/}}z = 71\):
\(m{\text{/}}z = 43\):
Suggest a reason for the peak at m/z = 43 having an exceptionally high relative abundance.
▶️Answer/Explanation
Markscheme
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\);
Accept full or condensed structural formulas.
Ignore incorrect names as long as structures are correct.
same/similar (types of) bonds / both contain the carbonyl group/C=O;
Do not accept same functional group.
(mass spectrum of) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\) contains peak at \(m{\text{/}}z = 29\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) does not contain peak at \(m{\text{/}}z = 29\);
(corresponding to) loss of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\) / \({M_{\text{r}}} – {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\) / \({\text{CH}}{{\text{O}}^ + }\) / loss of CHO / \({M_{\text{r}}} – {\text{CHO}}\) / \({{\text{C}}_{\text{2}}}{\text{H}}_{\text{5}}^ + \);
OR
(mass spectrum of) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\) contains a (strong) peak at \(m{\text{/}}z = 57\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) does not contain a (strong) peak at \(m{\text{/}}z = 57\);
(corresponding to) loss of H / \({M_{\text{r}}} – {\text{H}}\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}^ + }\);
Penalize missing + once only in A1.
m/z = 86: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCH}}_{\text{3}}^ + {\text{/}}{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{COCH}}_{\text{3}}^ + {\text{/}}{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}^ + }\);
m/z = 71: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{C}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{{\text{O}}^ + }\);
Accept CH3COCH2CH2+
m/z = 43: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}}_{\text{2}}^ + {\text{ / C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }{\text{ / }}{{\text{C}}_{\text{3}}}{\text{H}}_{\text{7}}^ + {\text{ / }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
Penalize missing + once only in A1.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}}_{\text{2}}^ + \) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\)/two species have this mass/m/z;
Do not penalize missing + in this part.
Examiners report
The majority of candidates was able to identify the two structures in (a) and recognized that IR spectroscopy could not distinguish them easily because they contained the same types of bonds in (b).
The majority of candidates was able to identify the two structures in (a) and recognized that IR spectroscopy could not distinguish them easily because they contained the same types of bonds in (b).
Answers to part (c) were often general and did not meet the requirements. Only few candidates predicted the peaks in the mass spectrum that could be used to distinguish the two compounds.
Part (d)(i) and (ii) were answered well by about half the candidates. However, some candidates are still forgetting to include a positive charge for fragments detected in the mass spectrometer.
Part (d)(i) and (ii) were answered well by about half the candidates. However, some candidates are still forgetting to include a positive charge for fragments detected in the mass spectrometer.
Question
The low resolution \(^1{\text{H}}\,{\text{NMR}}\) spectrum of compound Q is shown.
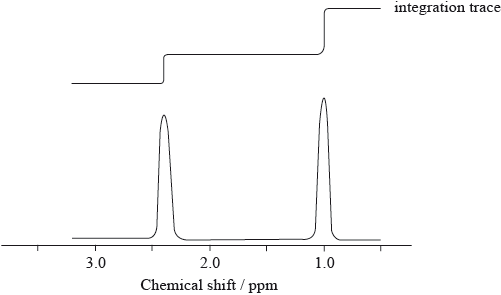
Identify what information from the spectrum allows the determination of the relative numbers of hydrogen atoms producing each peak.
Deduce which of the following compounds is Q.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\quad \quad \quad \quad \quad {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\quad \quad \quad \quad \quad {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\]
Identify the wavenumbers of two peaks in the infrared spectrum of compound Q, using Table 17 of the Data Booklet.
▶️Answer/Explanation
Markscheme
(ratio of) area under each peak / integration trace;
Accept size of peak but not height of peak.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\);
1700–1750 \({\text{(c}}{{\text{m}}^{ – 1}}{\text{)}}\) and 2850–3100 \({\text{(c}}{{\text{m}}^{ – 1}}{\text{)}}\);
Accept a single value or a smaller range within each range.
Examiners report
This was a well-answered question generally. In part (a) most candidates recognized the area under the peak as the indicator of the number of hydrogen atoms in NMR spectroscopy, although few candidates thought it was the chemical shift, and few candidates incorrectly stated that it was the height of the peak (rather than the area underneath it).
This was a well-answered question generally. The majority of candidates chose the correct molecule (pentan-2-one) in part (b) and gave the correct ranges of IR absorptions in part (c).
This was a well-answered question generally. The majority of candidates chose the correct molecule (pentan-2-one) in part (b) and gave the correct ranges of IR absorptions in part (c).
Question
Three isomers of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\) are ethyl methanoate, methyl ethanoate and propanoic acid.
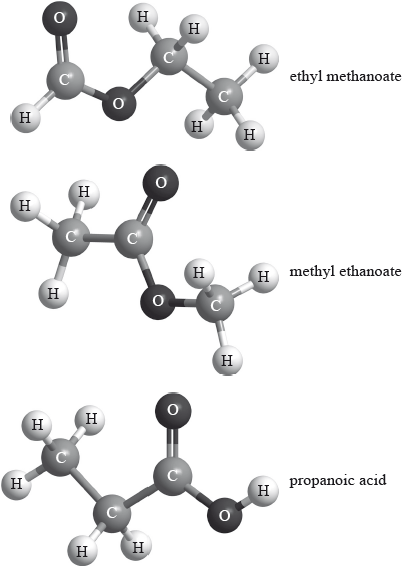
Explain which of the three compounds has a mass spectrum which contains peaks at \(m{\text{/}}z = 59\) and 44.
▶️Answer/Explanation
Markscheme
methyl ethanoate;
peak at 44 corresponds to loss of two methyl/CH3 groups (and peak at 59 the loss of the first) / the only isomer with two methyl groups / OWTTE;
Examiners report
Many students could use the spectroscopic data provided to correctly identify the compounds, with the first part of the question, dealing with mass spectrometer data, probably proving the most challenging.
Question
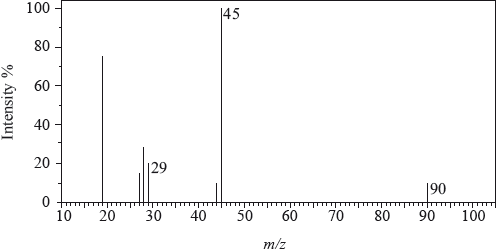
The mass spectrum of an unknown acidic compound, X, with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\), is shown below.
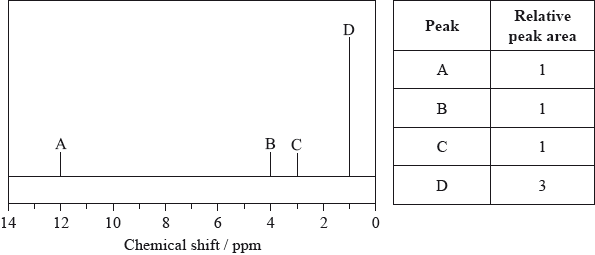
The low-resolution \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows four peaks. A simplified representation is shown alongside a table with relative peak areas.
Determine the relative molecular mass, to the nearest integer, of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formula of the fragment responsible for the peak at 45.
Deduce the formula of the fragment responsible for the peak at 29.
Identify the group responsible for the peak at D.
Suggest a possible structure for X.
▶️Answer/Explanation
Markscheme
90;
\({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_3^ + \);
Penalize missing positive charge of ion only once in (a).
\({\text{COO}}{{\text{H}}^ + }\);
Accept C2H5O+.
Penalize missing positive charge of ion only once in (a).
\({\text{CH}}{{\text{O}}^ + }/{\text{CO}}{{\text{H}}^ + }\);
Accept C2H5+/CH3CH2+.
Penalize missing positive charge of ion only once in (a).
\({\text{C}}{{\text{H}}_3}\)/methyl;
\({\text{C}}{{\text{H}}_3}{\text{CH(OH)COOH}}\);
Allow full or condensed structural formula.
Examiners report
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
Question
Describe the essential difference between the emission spectrum of sodium and the absorption spectrum of sodium.
Identify the five missing components in the following table.
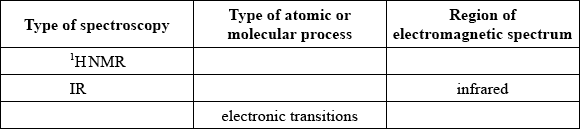
▶️Answer/Explanation
Markscheme
Emission spectrum: coloured lines and Absorption spectrum: black/dark lines;
OR
Emission spectrum: lines and Absorption spectrum: continuous;
Allow “Emission spectrum: electrons emit energy as they drop to lower energy levels and Absorption spectrum: electrons absorb energy as they are promoted to higher energy levels” / OWTTE.
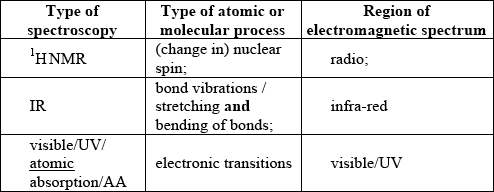 ;
;
For M4 both visible/UV/atomic absorption/AA for type of spectroscopy and region of EMS required.
Examiners report
Students found it difficult to explain clearly the difference between emission and absorption spectra for part (a). Most candidates successful in gaining the mark, described the difference in terms of energy released or absorbed by the electrons. There was lack of understanding however for the difference in the coloured and the dark lines produced by the two spectra. Candidates who described the difference in terms of coloured lines or continuous spectrum had difficulty attaining the mark. Very few students achieved all 4 points for part (b). Most had difficulty completing the information for \(^{\text{1}}{\text{H}}\,{\text{NMR}}\). Many stated a number for what was measured instead of the atomic/molecular process e.g. chemical shift as opposed to nuclear spin. Candidates were well prepared for answering part (c) often, stating concentration of the element as the answer.
Students found it difficult to explain clearly the difference between emission and absorption spectra for part (a). Most candidates successful in gaining the mark, described the difference in terms of energy released or absorbed by the electrons. There was lack of understanding however for the difference in the coloured and the dark lines produced by the two spectra. Candidates who described the difference in terms of coloured lines or continuous spectrum had difficulty attaining the mark. Very few students achieved all 4 points for part (b). Most had difficulty completing the information for \(^{\text{1}}{\text{H}}\,{\text{NMR}}\). Many stated a number for what was measured instead of the atomic/molecular process e.g. chemical shift as opposed to nuclear spin. Candidates were well prepared for answering part (c) often, stating concentration of the element as the answer.
Question
The mass spectrum of iodoethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{I}}\), shows three prominent peaks with m/z values of 156, 127 and 29. Identify the ions responsible for each of these three prominent peaks.
Bromine contains two isotopes, \(^{{\text{79}}}{\text{Br}}\) and \(^{{\text{81}}}{\text{Br}}\), in approximately equal amounts. Predict the m/z values of the prominent peaks in the mass spectrum of bromoethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\).
▶️Answer/Explanation
Markscheme
\({{\text{C}}_2}{{\text{H}}_5}{{\text{I}}^ + }\);
\({{\text{I}}^ + }\) and \({{\text{C}}_2}{\text{H}}_5^ + \);
Penalize once only if the + sign is missing.
108 and 110;
79 and 81;
29;
Examiners report
Part (a), identification of the ions, was answered well by most candidates. Few missed charge or used incorrect charge on the ions e.g. \({{\text{I}}^ – }\). For part (b), most scored the point for 29. Prominent peaks values for bromine isotopes proved to be a little more challenging for the candidates, many gave average values of 109 and 80 as other answers.
Part (a), identification of the ions, was answered well by most candidates. Few missed charge or used incorrect charge on the ions e.g. \({{\text{I}}^ – }\). For part (b), most scored the point for 29. Prominent peaks values for bromine isotopes proved to be a little more challenging for the candidates, many gave average values of 109 and 80 as other answers.
Question
Two students were provided with three different isomers of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\).
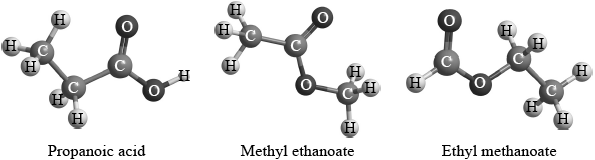
They were asked to suggest how the isomers could be distinguished and positively identified from each other using spectroscopic techniques. Student A said that they could be positively identified just from their infrared spectra. Student B said that they could be positively identified just from the number of peaks and the areas under each peak in their \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra.
Evaluate these two claims and suggest how any possible limitations could be overcome using the same spectroscopic technique.
Student A / Infrared:
Student B / \(^{\text{1}}{\text{H}}\,{\text{NMR}}\):
▶️Answer/Explanation
Markscheme
Student A / Infrared
propanoic acid can be distinguished from the other two by the (broad) absorption at \({\text{2500–3300 c}}{{\text{m}}^{ – 1}}\) /due to –OH absorption in carboxylic acids;
not easy to distinguish between methyl ethanoate and ethyl methanoate because all absorb at \({\text{1700–1750 c}}{{\text{m}}^{ – 1}}\) / C=O / \({\text{1050–1410 c}}{{\text{m}}^{ – 1}}\) / C–O / \({\text{2850–3100 c}}{{\text{m}}^{ – 1}}\) / C–H / because they have same functional groups;
can be distinguished from the pattern in the fingerprint region / by comparing with spectra of known samples;
Student B / 1H NMR
methyl ethanoate can be distinguished from the other two as it will have two peaks of equal area (due to the two –CH3 groups);
propanoic acid and ethyl methanoate cannot be distinguished as both have three peaks / peaks in the ratio of 1:2:3;
chemical shift is also required to distinguish them;
Absorption value or name of functional group required for M1 and M2.
Examiners report
This question proved to be very challenging for most candidates as majority were unable to evaluate the two claims. Candidates appeared to have some general understanding but were often lacking depth in understanding of the two analytical techniques. The answers were often too general and most did not suggest ways to overcome limitations using the same spectroscopic techniques. Limited understanding of the directive term ‘evaluate’ along with missing the ‘same’ spectroscopic technique in the question stem, penalized the most students from scoring even part marks.
Question
Compound X has the molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{3}}}\) and is found in human perspiration.
Y is an isomer of X, which contains the same functional groups.
Its infrared (IR) spectrum is represented below.
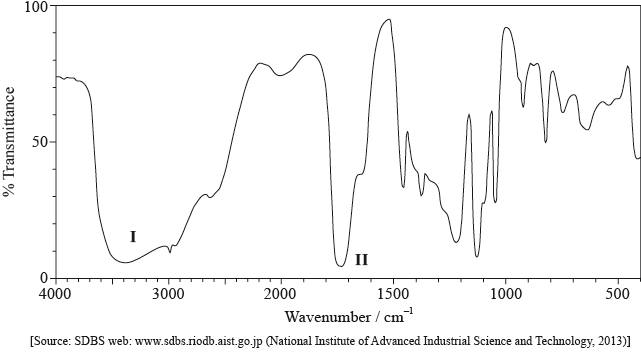
Deduce the bonds responsible for the absorptions labelled I and II.
I:
II:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the following chemical shift values (in ppm):
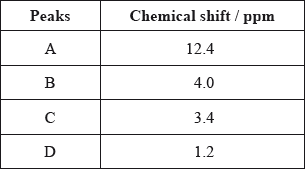
The integration trace for A:B:C:D was found to be 1:1:1:3.
Deduce what information can be obtained about the hydrogen atoms responsible for peak D at 1.2 ppm from the integration trace in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X.
Deduce the fragments in the mass spectrum which correspond to the following m/z values.
m/z = 45:
m/z = 17:
m/z = 15:
Deduce the structural formula of X.
(i) Deduce the structural formula of Y.
(ii) Predict one difference between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of Y and X.
(i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the m/z value for the molecular ion peak on the mass spectrum of this compound.
(ii) Deduce the number of different chemical environments of the hydrogen atoms in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 3-methylbutanoic acid.
▶️Answer/Explanation
Markscheme
I: O–H and II: C=O;
Do not allow CO for C=O.
Allow OH for O–H.
three hydrogens in same (chemical) environment / \({\text{C}}{{\text{H}}_{\text{3}}}\)/methyl (group);
Award [2] for all three correct, [1] for any two correct.
m/z = 45:
\({\text{COO}}{{\text{H}}^ + }/{\text{C}}{{\text{O}}_2}{{\text{H}}^ + }/{{\text{C}}_2}{{\text{H}}_5}{{\text{O}}^ + }\);
m/z = 17:
\({\text{O}}{{\text{H}}^ – }\);
m/z = 15:
\({\text{CH}}_3^ + \);
Penalize missing + once only.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)COOH}}/{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
(i) \({\text{C}}{{\text{H}}_{\text{2}}}{\text{(OH)C}}{{\text{H}}_{\text{2}}}{\text{COOH}}/{\text{HO(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
(ii) different integration trace / integration trace 1:2:2:1 (in Y) / different chemical shift values / OWTTE;
(i) 102;
(ii) 4;
Examiners report
Most candidates scored this mark by identifying the bonds responsible for the absorptions.
About half the candidates were able to analyze the integration trace correctly and deduced that this was a methyl group.
Generally well answered. However, a few candidates are still forgetting to include the positive charge of the fragments of the mass spectrum.
About a third of the candidates were able to deduce the correct structural formula of X based on the evidence presented.
(i) Only few candidates deduced the correct structure for the isomer Y.
(ii) About half the candidates predicted a reasonable difference between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of X and Y.
(i) More than half the candidates were able to deduce the molecular formula from the name and hence calculated the m/z value of the molecular ion peak correctly.
(ii) More than half of the candidates deduced the correct number of chemical environments in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 3-methylbutanoic acid.
Question
The mass spectrum and infrared (IR) spectrum of a compound are shown below.
(i) State the information about this particular compound that can be derived from the mass spectrum and outline how it is found.
(ii) Suggest how the fragment with m/z = 43 is formed from the original molecule.
(i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ – 1}}\) to deduce one functional group that is present in the compound and one group that is absent.
Present:
Absent:
(ii) The molecular formula of the compound is \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\). Explain, with reference to another region of the IR spectrum, why the compound could not be propanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\).
(iii) Deduce the structures of two possible isomers of propanoic acid consistent with the IR spectrum.
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing between closely related compounds such as those above.
(i) State the region of the electromagnetic spectrum that is used in this technique.
(ii) The structures of two other closely related compounds are shown below.
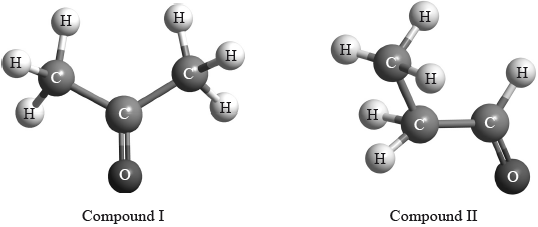
Discuss how you would expect the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of these two compounds to differ, using Table 18 of the Data Booklet.
▶️Answer/Explanation
Markscheme
(i) molar/molecular mass/\(M = 74{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\) / relative molecular mass/\({M_r} = 74\);
peak with highest m/z (ignoring any peak attributable to \(^{{\text{13}}}{\text{C}}\)) /
found from parent/molecular ion peak;
Allow mass for m/z.
OR
compound has methyl/\({\text{C}}{{\text{H}}_{\text{3}}}\);
m/z = 15 due to \({\text{CH}}_3^ + \);
OR
compound has propyl/\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)/isopropyl/\({\text{CH(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)/acetyl/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}\);
m/z = 43 due to \({{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + {\text{/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\);
OR
compound has acetoxy/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}\);
m/z = 59 due to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ + }\);
Fragment must contain + sign in relevant marks above.
Penalize missing charges where relevant once only in (a)(i) and (ii).
(ii) loss of \({\text{C}}{{\text{H}}_3}\)–O / loss of radical with m/z = 31 / formation of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + \);
Penalize missing charges where relevant once only in (a)(i) and (ii).
(i) Present: carbonyl/C=O;
Do not accept aldehyde / ketone.
Accept ester/alkanoate only if m/z = 59 given in (a)(i).
Absent: carbon-carbon double bond/C=C/alkene;
(ii) no (broad) absorption at 2500-3300 \({\text{(c}}{{\text{m}}^{ – 1}}{\text{)}}\);
no O–H bond;
Award [1 max] for just stating “absorption at 1050-1410 (cm–1) / C-O bond present of alcohol/ester/ether”.
Do not accept just “C-O bond present”.
Accept “peak” for “absorption”.
(iii) Any two structures from:
Do not penalize CH3-C connectivity here.
(i) radio (wave);
(ii) I: one signal/H environment and (compound) II: three signals/H environments;
EITHER
Award [1 max] for any two of the following chemical shift ranges from the Data Booklet:
I: 2.2-2.7 (ppm) / II: 2.2-2.7 (ppm)
II: 0.9-1.0 (ppm)
II: 9.4-10.0 (ppm);
Ranges must be associated with the correct compound (I or II).
OR
II: signal at 0.9-1.0 (ppm) and I: no such signal;
OR
II: signal at 9.4-10.0 (ppm) and I: no such signal;
Accept answers that correctly discuss differences in splitting patterns (though not on SL syllabus).
Accept “peak” for “signal”.
Do not award M2 if any contradictory chemical shift ranges are given (eg, do not allow 0.9-1.0 (ppm) for compound I).
Examiners report
In (a) (i) many candidates associated the molecular ion peak with the molar mass of the compound. Some stated incorrectly that the mass was 74, instead of stating that the molar mass was \({\text{74 g}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). (ii) proved problematic for a number of candidates and the difference between the loss of radicals and the positively charged fragments remaining was often lost. Greater emphasis on this difference in the teaching of this part of the curriculum on fragmentation might be worth exploring further with cohorts.
In (b) (i), most candidates identified the presence of the C=O and the absence of the C=C groups on the IR spectrum.
In (ii), although a large number of candidates stated that there is no broad absorption in the \({\text{2500 – 3300 c}}{{\text{m}}^{ – 1}}\) range, equating this to the absence of the OH bond in the IR spectrum, surprisingly some gave a more limited range of \({\text{3200 – 3600 c}}{{\text{m}}^{ – 1}}\) and associated this with the absence of hydrogen bonding. Although Table 17 of the Data Booklet does not refer specifically to the broad nature of the OH absorption in the \({\text{2500 – 3300 c}}{{\text{m}}^{ – 1}}\) range for carboxylic acids, it might be worth pointing this feature out in the teaching of IR spectroscopy, based on careful analysis of real spectral examples of carboxylic acids. In (iii), the most common mistake involved candidates drawing isomers including the OH group, which scored no marks. One G2 comment claimed that drawing an ester is not part of the syllabus. However, esters are mentioned explicitly in AS 10.1.11.
In (c) (i), nearly all identified the correct region of the EMS, namely radiowaves. (ii) proved to be a good discriminating question. Many knew that compound I had one hydrogen environment and compound II had three hydrogen environments, but only the better candidates scored the second mark. The most common mistake involved stating that compound I had a chemical shift range 0.9-1.0 ppm for the six methyl hydrogens. Many candidates clearly did not realise that the methyl groups in propanone are adjacent to the carbonyl, so hence have a higher value for the chemical shift, i.e. 2.2-2.7 ppm.
Question
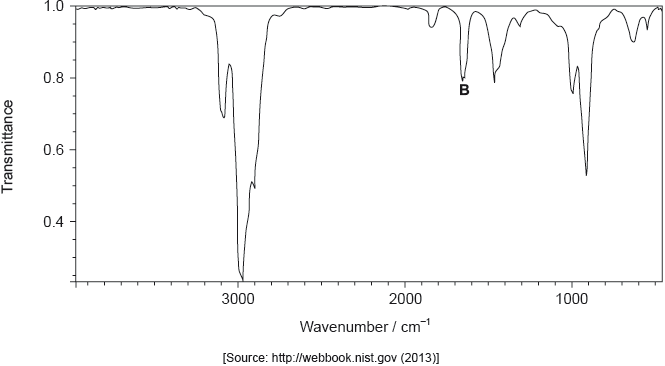
The structure of an unknown compound A with empirical formula CH2 can be determined using information from a variety of analytical techniques.
The mass spectrum of A is shown below.
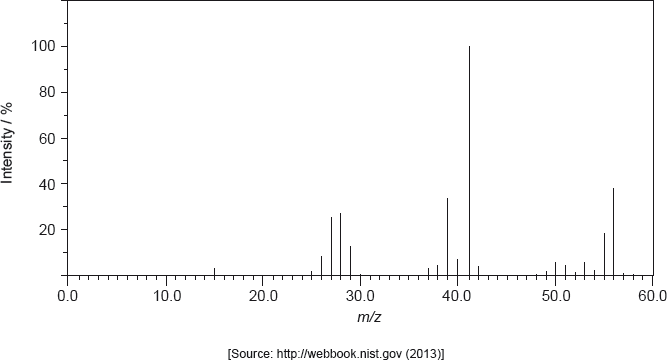
The infrared (IR) spectrum of A is shown below.
Determine the relative molecular mass of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formulas of the fragments which give rise to peaks at \(m/z = 27\) and 29.
\(m/z = 27\):
\(m/z = 29\):
Identify the bond responsible for the IR absorption at B.
Deduce a structural formula consistent with the data.
▶️Answer/Explanation
Markscheme
56;
\({{\text{C}}_{\text{4}}}{\text{H}}_{\text{8}}^ + \);
Penalize missing charge only once in (i) and (ii).
\(m/z = 27:{{\text{C}}_{\text{2}}}{\text{H}}_3^ + /{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}^ + }/{\text{C}}{{\text{H}}_{\text{2}}}\)=\({\text{C}}{{\text{H}}^ + }\) and \(m/z = 29:{{\text{C}}_{\text{2}}}{\text{H}}_{\text{5}}^ + /{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}_{\text{2}}^ + {\text{;}}\)
Penalize missing charge only once in (i) and (ii).
C=C/carbon−carbon double bond;
Accept “alkenyl/alkene”.
CH3CH2CH=CH2;
Accept either a full or a condensed structural formula.
Examiners report
More than half of the candidates obtained the molecular mass from the spectrum. About a third of the candidates identified \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) as the molecular formula but only a few candidates remembered to include the positive charge on the molecular ion and scored the second mark.
About a third of the candidates identified the correct fragments. It was disappointing to see candidates suggesting fragments that did not match the masses of the peaks.
Very well answered.
Only a few candidates deduced the correct structural formula consistent with the data.
Question
NMR spectroscopy is one of the most powerful analytical tools for determining molecular structure.
The 1H NMR spectrum, including the integration trace, of a ketone with relative molecular mass 86 is shown below.
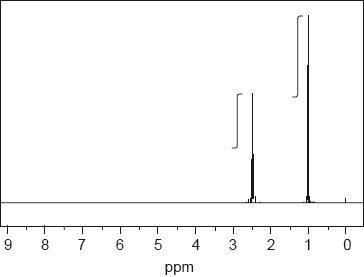
[Source: SDBS web: www.sdbs.riodb.aist.go.jp (National Institute of
Advanced Industrial Science and Technology, 2014)]
Deduce the structural formula of the compound, justifying your choice.
▶️Answer/Explanation
Markscheme
CH3CH2COCH2CH3;
Accept full or condensed structural formula.
Any two for [2 max] from:
two H (atom)/proton environments;
3:2/2:3 ratio of atoms in these environments;
one environment an alkyl group / one environment next to a carbonyl;
Accept –CH3 (0.9–1.0 ppm) / –CO–CH2– (2.2–2.7 ppm)
Examiners report
Many candidates did elicit the correct structure although the most common error was the incorrect positioning of the carbonyl group. The number of H environments was often established but the ratios of atoms was not seen very often. Many did score a mark for stating a correct shift associated with the methyl group.
Question
Consider the compound chloroethene, CH2=CHCl.
Deduce two features you would expect to observe in its mass spectrum.
Predict two features you would expect to observe in its infrared (IR) spectrum.
▶️Answer/Explanation
Markscheme
peaks at (m/z =) 62 and 64 / two molecular ion peaks;
height ratio of molecular ion peaks is 3 :1;
peak at (m/z =) 27 (due to loss of Cl);
Accept peaks at 63/65 (13C)/peak(s) resulting from 13C / peaks at 61/63 (loss of H)/peak(s) resulting from loss of H / peaks at 48/50 (loss of CH2)/peak(s) resulting from loss of CH2/ 35/37 Cl+ as an alternative to final marking point.
absorption at 600 – 800 (\({\text{c}}{{\text{m}}^{ – 1}}\) C–Cl);
absorption at 1610 – 1680 (\({\text{c}}{{\text{m}}^{ – 1}}\) >C=C<);
absorption at 2850 – 3100 (\({\text{c}}{{\text{m}}^{ – 1}}\) C–H);
Examiners report
The first and second marking points were rarely scored as most students did not recall that chlorine exists as a mixture of isotopes. The most common molecular ion peak stated was 62.5 instead of both 62 and 64. A common incorrect response in this question was to suggest fragmentation at the double bond with the production of the CH2 fragment. The identification of possible IR absorption bands and the effect of IR radiation being absorbed by molecules was well done by most candidates. It was clear that most candidates understood that bond stretching/bending occurred when IR radiation is absorbed but there were fewer correct references to the change in bond polarity which was required for the second mark.
The first and second marking points were rarely scored as most students did not recall that chlorine exists as a mixture of isotopes. The most common molecular ion peak stated was 62.5 instead of both 62 and 64. A common incorrect response in this question was to suggest fragmentation at the double bond with the production of the CH2 fragment. The identification of possible IR absorption bands and the effect of IR radiation being absorbed by molecules was well done by most candidates. It was clear that most candidates understood that bond stretching/bending occurred when IR radiation is absorbed but there were fewer correct references to the change in bond polarity which was required for the second mark.
Question
Opiates have been used for thousands of years to alleviate pain. The structures of opiates are found in section 37 of the data booklet.
Diamorphine (heroin) can be synthesized from morphine. Identify the reagent necessary for this reaction and the by-product of this reaction.
The reaction can be monitored by infrared spectroscopy. Using section 26 of the data booklet, identify two IR absorbance ranges that would help distinguishing the two compounds.
Present in morphine but not in diamorphine:
Present in diamorphine but not in morphine:
Discuss how the differences in structure between morphine and diamorphine affect their absorption in the body.
▶️Answer/Explanation
Markscheme
Accept names or structural formulas for reagent and by-product.
Accept IUPAC or alternative names of compounds e.g. acetic acid.
Award M2 only if the by-product corresponds to the reagent.
Present in morphine but not in diamorphine:
«has OH and absorbance at» 3200–3600 «cm–1»
Present in diamorphine but not in morphine:
«has C=O and absorbance at» 1700–1750 «cm–1»
morphine has «two» hydroxyl «groups» AND diamorphine/heroin has «two»
ester/ethanoate/acetate «groups»
morphine is more polar than diamorphine/heroin
diamorphine/heroin crosses the blood-brain barrier «easily»
morphine is soluble in blood «plasma»
OR
diamorphine/heroin is «more» soluble in lipids
Accept converse argument throughout. Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Do not accept “diamorphine/heroin is non-polar” for M2.
Question
Infrared (IR) spectroscopy is often used for the identification of polymers, such as PETE, for recycling.
LDPE and high density polyethene (HDPE) have very similar IR spectra even though they have rather different structures and physical properties.
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
Describe the difference in their structures.
Explain why the difference in their structures affects their melting points.
▶️Answer/Explanation
Markscheme
A RIC: 1 AND B RIC: 4
ALTERNATIVE 1:
«only» PETE contains carbonyl/C=O/ester/COO groups
carbonyl groups absorb at 1700–1750 «cm–1»
ALTERNATIVE 2:
LDPE contains more C–H bonds «than PETE»
C–H bonds absorb at 2850–3090 «cm–1»
For either, accept specific frequencies in these ranges (eg 1735 «cm–1» or 2900 «cm–1»).
[3 marks]
HDPE less branched
OR
LDPE more branched
Accept “no branching in HDPE AND branching in LDPE”.
[1 mark]
HDPE «polymer» chains/molecules can pack together more closely «than LDPE chains»
OR
HDPE «polymer» chains/molecules have a higher contact surface area «than LDPE chains»
stronger intermolecular/dispersion/London/van der Waals’ forces in HDPE AND higher melting point
Accept converse arguments.
[2 marks]
Question
Solubility plays an important role in the bioavailability of drugs in the body.
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
▶️Answer/Explanation
Markscheme
presence of «large» benzene/arene ring AND non-polar/hydrophobic
OR
presence of «large» benzene/arene ring AND cannot form H-bond with water
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND polar/hydrophilic
OR
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND can form H-bonds with water
Accept “phenyl” for “benzene ring”.
Accept “carboxylic acid” for “carboxyl”.
Do not accept “alcohol” for “hydroxyl”.
[2 marks]
OR
C6H4(OCOCH3)COOH + NaOH → C6H4(OCOCH3)COONa + H2O
Charges (O– and Na+) not necessary to score the mark.
Accept net ionic equation.
Accept any strong base in place of NaOH.
[1 mark]
«student’s» sample impure
lattice disrupted/not uniform «due to presence of impurities»
OR
fewer interparticle/intermolecular forces «due to presence of impurities»
Accept converse arguments.
[2 marks]
One similarity:
peak at 2500–3000 «cm–1»/peak due to O–H/hydroxyl in carboxylic acids
OR
peak at 1700–1750 «cm–1»/peak due to C=O/carbonyl
OR
peak at 2850–3090 «cm–1»/peak due to C–H of arene
One difference:
peak at 3200–3600 «cm–1» in salicylic acid/ peak due to O–H in phenol in salicylic acid
OR
«two» peaks at 1700–1750 «cm–1» in aspirin AND one peak «in the same area» in salicylic acid
Accept “peak at 1600 cm–1 for arene/benzene ring” – not in the data booklet.
Accept “2500–3600 cm–1 «overlapping absorptions of two O–H» in salicylic acid”.
Accept “stronger/broader/split peak at 1700–1750 cm–1 in aspirin”.
[2 marks]
«use of» alternative solvents such as supercritical/liquid CO2
OR
use of water «as solvent»
OR
solvent-free reactions «for example, polymerization of propene»
OR
solid-state chemistry
OR
recycle «waste» solvents
OR
catalysis that leads to better/higher yield
OR
reducing number of steps
Do not accept political/regulatory solutions.
“catalysis” not sufficient for mark.
[1 mark]
Question
Infrared (IR) spectra can be used to distinguish between various types of plastics. Some simplified IR spectra are given here.
Explain, with a reference to molecular structure, which two of the plastics can not be distinguished by IR spectroscopy.
▶️Answer/Explanation
Markscheme
HDPE AND LDPE «have similar IR»
both are polyethene/polyethylene
OR
only branching differs
OR
same bonds
OR
same bending/stretching/vibrations
Accept “water bottle AND water bottle cap” for M1.
[2 marks]
Examiners report
Question
The structures of oseltamivir (Tamiflu) and zanamivir (Relenza) are given in section 37 of the data booklet.
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
Suggest one ethical consideration faced by medical researchers when developing medications.
▶️Answer/Explanation
Markscheme
One similarity:
both contain amido «group»
One difference:
oseltamivir contains ester «group» AND zanamivir does not
OR
oseltamivir contains amino «group» AND zanamivir does not «but contains a guanidino group»
OR
zanamivir contains carboxyl «group» AND oseltamivir does not
OR
zanamivir contains «several» hydroxyl «groups» AND oseltamivir does not
OR
oseltamivir contains ester «group» AND zanamivir contains carboxyl «group»
OR
oseltamivir contains ester «group» AND zanamivir contains «several» hydroxyl «groups»
Accept “both contain ether «group»” OR “both contain alkene/alkenyl «group»” OR “both contain carbonyl «group»” OR “both contain amino/amine «group»”. Latter cannot be given in combination with second difference alternative with respect to amino group.
Accept “amide/carboxamide/carbamoyl” for “amido”.
Accept “amine” for “amino”.
Accept “carboxylic acid” for “carboxyl”.
Accept “hydroxy/alcohol” for “hydroxyl”, but not “hydroxide”.
[2 marks]
1050–1410
OR
1620–1680
OR
1700–1750
OR
2500–3000
OR
3200–3600
OR
2850–3090
OR
3300–3500 «cm–1»
[1 mark]
«negative» side-effects of medication on patient/volunteers
OR
effects on environment «from all materials used and produced»
OR
potential for abuse
OR
drugs may be developed that are contrary to some religious doctrines
OR
animal testing
OR
risk to benefit ratio
OR
appropriate consent of patient volunteers
[1 mark]
Question
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
▶️Answer/Explanation
Markscheme
Any two of:
ability to form a LC phase
chemically stable
«LC phase that is» stable over suitable temperature range
polar
OR
being able to change orientation with applied electric field
rapid switching speed «responds to changes of voltage quickly»
Accept “ability of molecules to transmit light under certain conditions” OR “rodshaped molecules” OR “stable to light/not light sensitive”.
[Max 2 Marks]
branching in LDPE prevents close packing «of chains»
LDPE is more flexible/less rigid
OR
LDPE has lower «tensile» strength
Do not accept “difference in density”.
Award [1 max] for stating “branching in LDPE AND little/no branching in HDPE”.
B AND absence «of absorption of» C–H at 2850–3090 «cm–1»
OR
B AND presence of «absorption of» C–F at 1000–1400 «cm–1»
(–C2H3Cl–)2 (s) + 5O2 (g) → 4CO2 (g) + 2H2O (l) + 2HCl (g)
correct species in reactants and products
balanced
Accept “(–C2H3Cl–)2 (s) + 5.5O2 (g) → 4CO2 (g) + 3H2O (l) + Cl2 (g)”.
Award M2 only if M1 correct.
Question
Amino acids are the building blocks of proteins.
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
Deduce the number of 1H NMR signals produced by the zwitterion form of alanine.
Outline why amino acids have high melting points.
▶️Answer/Explanation
Markscheme
peptide bond
order of amino acids
Accept zwitterion form of dipeptide.
Accept a condensed structural formula or a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
[2 marks]
3
[1 mark]
form zwitterions
«strong» ionic bonding
OR
«strong» ionic lattice
OR
«strong» electrostatic attraction/forces
Do not accept hydrogen bonding or IMFs for M2.
[2 mark]

