Question
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
(a) The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1. [2]
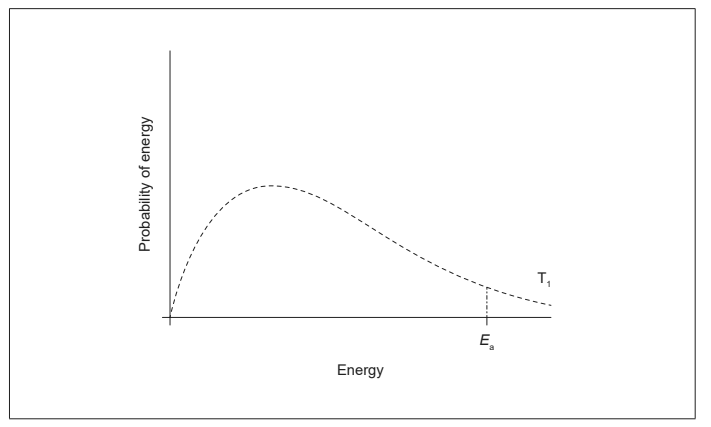
(b) The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product. [1]
(c) Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
(i) Determine the overall equation for the production of methanol. [1]
(ii) 8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet. [3]
(d) Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
(i) Determine the enthalpy change, ∆H, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol–1. [3]
(ii) State one reason why you would expect the value of ∆H calculated from the ∆H Θf values, given in section 12 of data booklet, to differ from your answer to (d)(i). [1]
(iii) State the expression for Kc for this stage of the reaction. [1]
(iv) State and explain the effect of increasing temperature on the value of Kc. [1]
(e) Now consider the second stage of the reaction.
CO (g) + 2H2 (g) CH3OH (l) ∆HΘ = –129 kJ
(i) The equilibrium constant, Kc, has a value of 1.01 at 298 K. Calculate ∆G Θ, in kJ mol–1, for this reaction. Use sections 1 and 2 of the
data booklet. [2]
(ii) Calculate a value for the entropy change, ∆S Θ, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer. [2]
(iii) Justify the sign of ∆S with reference to the equation. [1]
(iv) Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the spontaneity of the reaction. [1]
Answer/Explanation
Ans:
4. a
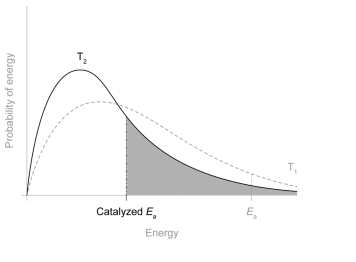
curve higher AND to left of T1 new/catalysed Ea marked AND to the left of Ea of curve T1
Do not penalize curve missing a label, not passing exactly through the origin, or crossing x-axis after Ea. Do not award M1 if curve drawn shows significantly more/less molecules/greater/smaller area under curve than curve 1. Accept Ea drawn to T1 instead of curve drawn as long as to left of marked Ea.
4.b
methanoic acid/HCOOH/CHOOH
OR methanal/HCHO Accept “carbon dioxide/CO2”.
4. c i
CH4(g) + H2O(g) + CH3OH(l) + H2(g)
Accept arrow instead of equilibrium sign.
4. c ii
amount of methane = « = » 0.498 «mol»
amount of hydrogen = amount of methane / 0.498 «mol»
volume of hydrogen = «0.498 mol » 22.7 dm3 mol−1 = » 11.3 «dm3»
Award [3] for final correct answer.
Award [2 max] for 11.4 «dm3 due to rounding of mass to 16/moles to 0.5. »
4. d i
∑bonds broken = 4 × 414 «kJ» + 2 × 463 «kJ» / 2582 «kJ»
∑bonds formed = 1077 «kJ» + 3 × 436 «kJ» / 2385 «kJ»
H «= ∑bonds broken − ∑bonds formed =( 2582 kJ − 2385 kJ)» = «+»197«kJ»
Award [3] for final correct answer.
Award [2 Max] for final answer of −197 «kJ»
4. d ii bond energies are average values «not specific to the compound»
4. d (iii) k2 = ![]()
4. d iv Kc increases AND «forward» reaction endothermic
4. e i
«ΔGƟ = − RTlnKc»
ΔGƟ = − 8.31 «J K−1 mol−1» × 298 «K» × ln (1.01) / −24.6 «J mol−1» = −0.0246 «kJ mol–1»
Award [2] for correct final answer. Award [1 max] for +0.0246 «kJ mol–1».
4. e ii
«ΔGƟ = ΔHƟ − TΔS Ɵ»
ΔGƟ = −129 «kJ mol–1» − (298 «K» × ΔS) = −0.0246 «kJ mol–1»
ΔS Ɵ = « = » −433 «J K–1 mol–1»
4. e iii «negative as» product is liquid and reactants gases OR fewer moles of gas in product
4. e iv
reaction «more» spontaneous/ΔG negative/less positive AND effect of negative entropy decreases/TΔS increases/is less negative/more positive
OR reaction «more» spontaneous/ΔG negative/less positive AND reaction exothermic «so Kc increases »
Question
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]

Determine the \(\Delta {S^\Theta }\) for the combustion of methanol.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\][2]
Using the enthalpy of combustion for methanol from Table 12 of the Data Booklet and the \(\Delta {S^\Theta }\) determined in part (d), calculate the standard free energy change for the combustion of methanol.[3]
Explain whether changing the temperature will alter the spontaneity of the reaction.[1]
Answer/Explanation
Markscheme
\(\Delta {S^\Theta }\left( { = \sum {S_{{\text{products}}}^\Theta } – \sum {S_{{\text{reactants}}}^\Theta } } \right) = 2 \times 189 + 214 – (240 + 1.5 \times 205)\);
\( = 44.5{\text{ J}}\,{{\text{K}}^{ – 1}}{\text{mo}}{{\text{l}}^{ – 1}}/0.0445{\text{ kJ}}\,{{\text{K}}^{ – 1}}{\text{mo}}{{\text{l}}^{ – 1}}\)
Award [2] for correct final answer.
Do not award M2 if M1 incorrect.
temperature of 298 K;
\(\Delta G_{\text{c}}^\Theta = {\text{(}}\Delta H_{\text{c}}^\Theta – T\Delta S_{\text{c}}^\Theta = ) – 726 – 298 \times 44.5 \times {10^{ – 3}}{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}/\)
\( = – 726\,000 – 298 \times 44.5{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
\( = – 739{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}/ – 7.39 \times {10^5}{\text{ J}}\,{\text{mo}}{{\text{l}}^{ – 1}}\);
Award [3] for correct final answer.
\(\Delta G_{\text{c}}^\Theta \) is always negative and temperature won’t alter spontaneity of reaction;
Examiners report
(d) caused difficulties for candidates with the multiplication factor for the oxygen and the correct units were frequently omitted which caused problems then subsequently in part (e).
In (e), problems of units were widespread.
(f) usually was well done by the better candidates. One G2 comment also stated that it would have been better if the experimental value would have been closer to the expected value.
