Question
Liquid-crystal displays (LCDs) have many uses.
A molecule which acts as a chiral nematic thermotropic liquid-crystal is given.
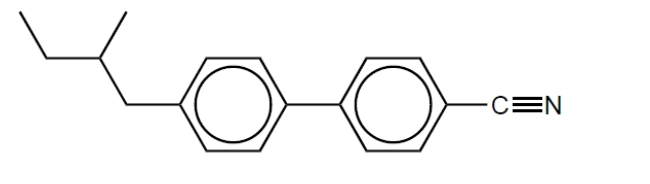
a. Label with an asterisk, ${ }^*$, the chiral carbon atom.
b. Explain the effects of very low and high temperatures on the liquid-crystal behaviour of this molecule.
Low temperature:
High temperature:
▶️Answer/Explanation
Markscheme
a.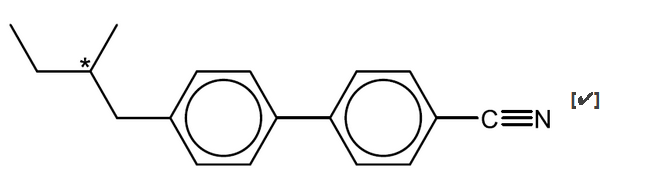
b. Low temperature:
intermolecular forces prevent molecules moving AND solid/«normal» crystal formation [ $[$ ]
High temperature:
«above a critical temperature» disrupts alignment of molecules AND behaves as fluid/liquid [ $\boldsymbol{V}$ ]
Note: Accept “weak intermolecular forces break AND behaves as fluid/liquid”.
Question
a. State the feature of Taxol that is a major challenge in its synthesis. Use section 37 of the data booklet.
b. Describe how the challenge in (a) was resolved by pharmaceutical companies.
▶️Answer/Explanation
Markscheme
a. numerous stereoisomers/chiral carbons/chiral centres/stereocentres/optical isomers
NOTE: Accept exact number of chiral carbons ie 11, but do not accept just “chiral”.
b. chiral auxiliaries/molecule binds to reactant blocking one reaction site «by steric hindrance»
OR
asymmetric synthesis / enantioselective catalysis «producing a specific enantiomer»
OR
biosynthesis / genetically modified bacteria/microorganisms
NOTE: Accept “use of a chiral auxiliary leading to “the synthesis of” the desired enantiomer”.
Question
Different conventions are used to classify enantiomers. Orange and lemon peel each contain different enantiomers of the compound limonene. One of the enantiomers is represented below.
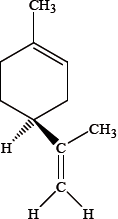
Identify the chiral centre in this enantiomer with an asterisk, *.[1]
The \(( + )d\)-enantiomer has the characteristic smell of oranges and the \(( – )l\)-enantiomer has the characteristic smell of lemons. Explain the meaning of the \(( + )d\) and \(( – )l\) symbols used in this notation.[1]
The R, S notation is also used. The \(( + )d\)-enantiomer is often described as R-limonene and the \(( – )l\)-enantiomer as S-limonene. Explain what is meant by the R, S notation and state whether the structure shown is R or S.[2]
▶️Answer/Explanation
Markscheme
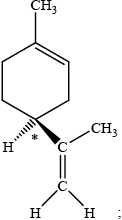
dextro/\(d\) and levo/\(l\) refer to right and left-handed / clockwise and anti-clockwise rotation of plane polarized light;
clockwise and anti-clockwise sequence of prioritized atoms (working from high to low atomic numbers) / OWTTE;
Allow absolute configuration of enantiomers.
Allow convention for labelling chiral carbon atoms using the Cahn-Ingold-Prelog notation.
S;
Examiners report
In (a), surprisingly a number of students were not able to identify the chiral centre.
Although some knew what \(d\) and \(l\) referred to, a number did not refer to plane-polarized light.
R and S notation was not understood and only the best candidates were able to determine the structure as S. Some simple class exercises on chiral centres, \(d\) and \(d\) notation and R and S notation using a number of simple molecules would greatly improve student understanding of this topic.
