Question
The standard electrode potentials for three other electrode systems are given below.

Using Table 14 of the Data Booklet, calculate the cell potential for this cell.[2]
The standard electrode potentials for three other electrode systems are given below.

(i) Identify which species in the table above is the best reducing agent.
(ii) Deduce the equation for the overall reaction with the greatest cell potential.[3]
These values were obtained using a standard hydrogen electrode. Describe the materials and conditions used in the standard hydrogen electrode. (A suitably labelled diagram is acceptable).[4]
Two electrolytic cells are connected in series as shown in the diagram below. In one there is molten magnesium chloride and in the other, dilute sodium hydroxide solution. Both cells have inert electrodes. If 12.16 g of magnesium is produced in the first cell, deduce the identity and mass of products produced at the positive and negative electrodes in the second cell.
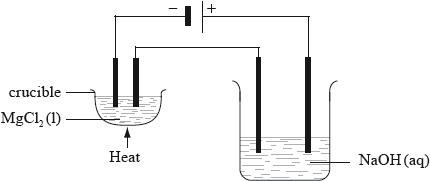 [4]
[4]
Answer/Explanation
Markscheme
\( + 0.80 – ( – 2.37) = 3.17{\text{ V}}\)
correct data;
answer with unit;
Award [1] for –3.17 V or correct working of wrong values.
(i) Cd/Cd(s);
Do not allow Cd2+.
(ii) \({\text{5Cd(s)}} + {\text{2MnO}}_4^ – {\text{(aq)}} + {\text{16}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
balancing;
If wrong equation is given, award [1] for correct balancing.
Ignore state symbols.
Accept suitable diagram with the following indicated:
Pt electrode;
\({\text{[}}{{\text{H}}^ + }{\text{(aq)]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{/0.5 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{ }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\);
Accept Molar, M or mol L–1 instead of mol\(\,\)dm–3.
\({{\text{H}}_{\text{2}}}\) gas;
at 1 atm / \(1.01 \times {10^5}{\text{ Pa}}\);
Do not award mark for pressure if no hydrogen gas given.
Products:
oxygen at positive electrode and hydrogen at negative electrode;
Moles of \({\text{Mg}} = 0.5\) / mole ratio of \({{\text{O}}_{\text{2}}}:{{\text{H}}_{\text{2}}}\) is \(1:2\);
Can be implied by calculation.
mass oxygen \( = \left( {\frac{1}{2} \times \frac{{12.16}}{{24.31}} \times 32.00 = } \right){\text{ }}8.00{\text{ g}}\);
mass hydrogen \( = \left( {\frac{{12.16}}{{24.31}} \times 2.02 = } \right){\text{ }}1.01{\text{ g}}\);
Do not apply SD rule here.
Examiners report
Attempts at the equations were disappointing, with many written the wrong way round, or not identified as oxidation or reduction and some candidates gave equilibrium signs.
In (b)(i) the most common mistake was stating \({\text{C}}{{\text{d}}^{2 + }}\) instead of Cd. In (b) (ii) many candidates were tempted to write the more familiar equation involving \({\text{F}}{{\text{e}}^{2 + }}\) and \({\text{MnO}}_4^ – \) ions even if they identified Cd.
The hydrogen electrode did not seem to be well known, with few diagrams resembling the real thing, rarely were all 4 marks scored – often candidates mentioned 1 atm but not \({{\text{H}}_{\text{2}}}{\text{(g)}}\), or \({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) but not \({{\text{H}}^ + }\).
The calculation was poorly done – in most cases it involved the wrong products, which usually included sodium, very few candidates realised that the products would be hydrogen and oxygen.
Question
The word redox comes from a combination of the terms reduction and oxidation. Redox reactions affect our daily lives.
The overall reaction that takes place in a voltaic cell is shown below.
\[{\text{Pb(s)}} + {\text{Pb}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}} \to {\text{2PbS}}{{\text{O}}_{\text{4}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Use information from Table 14 of the Data Booklet to deduce the oxidizing agent that can oxidize chloride ions but not fluoride ions. State the redox equation for the reaction and determine its cell potential.[4]
(i) Molten sodium chloride is electrolysed in a cell using inert electrodes. State the half-equation, with state symbols, for the reaction taking place at the positive electrode (anode) and for the reaction taking place at the negative electrode (cathode). Determine the mole ratio of the products formed.
(ii) Predict and explain the products of electrolysis of a concentrated solution of NaCl (aq) using inert electrodes. Your answer should include half-equations with state symbols for the reaction at each electrode.[7]
Electroplating is an important application of electrolytic cells with commercial implications. Copper may be plated using an electrolytic cell with an aqueous acidified copper(II) sulfate electrolyte.
For the copper plating of tin to make jewellery, state the half-equation at each electrode. Assume the other electrode is also inert. Suggest two observations that you would be able to make as the electroplating progresses.[4]
Answer/Explanation
Markscheme
\({\text{MnO}}_4^ – \);
\({\text{2MnO}}_4^ – {\text{(aq)}} + {\text{16}}{{\text{H}}^ + }{\text{(aq)}} + {\text{10C}}{{\text{l}}^ – }{\text{(aq)}} \to {\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(l)}} + {\text{5C}}{{\text{l}}_2}{\text{(g)}}\)
Accept equation with all coefficients divided by 2 (i.e.
MnO4– + 8H+ + 5Cl– \( \to \) Mn2+ + 4H2O + 2\(\frac{1}{2}\)Cl2).
Award [1] for correct reactants and products, [1] for correct balancing.
Ignore state symbols.
\(E_{{\text{cell}}}^\Theta = (1.51 – 1.36) = ( + )0.15{\text{ (V)}}\);
(i) Positive/+/anode
\({\text{2C}}{{\text{l}}^ – }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ – }\);
Negative/–/cathode
\({\text{N}}{{\text{a}}^ + }{\text{(l)}} + {{\text{e}}^ – } \to {\text{Na(l)}}\);
Penalize missing or incorrect states such as (aq) or (s) once only.
Award only [1] if electrodes not specified or if equations switched.
\({\text{1C}}{{\text{l}}_2}\) to 2Na;
(ii) (choice of \({\text{C}}{{\text{l}}^ – }\) or \({{\text{H}}_2}{\text{O}}/{\text{O}}{{\text{H}}^ – }\) to be oxidized), \({\text{C}}{{\text{l}}^ – }\) oxidized because of concentrated solution/higher concentration / OWTTE;
(choice of \({\text{N}}{{\text{a}}^ + }\) or \({{\text{H}}_{\text{2}}}{\text{O}}/{{\text{H}}^ + }\) to be reduced), \({{\text{H}}_{\text{2}}}{\text{O/}}{{\text{H}}^ + }\) reduced because \({\text{N}}{{\text{a}}^ + }\) is a (much) weaker oxidizing agent/ \({\text{N}}{{\text{a}}^ + }\) not reduced to Na in water / \({{\text{H}}^ + }\) easier to reduce than \({\text{N}}{{\text{a}}^ + }\) / OWTTE;
positive/+/anode
\({\text{2C}}{{\text{l}}^ – }{\text{(aq)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ – }\);
negative/–/cathode
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ – } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ – }{\text{(aq) / 2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \to {{\text{H}}_2}{\text{(g)}}\);
Penalize missing or incorrect states once only.
Award only [1] out of the last two marks if electrodes not specified or if equations switched.
Positive/+/anode
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ – }/{{\text{H}}_2}{\text{O(l)}} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ – }/\)
\({\text{4O}}{{\text{H}}^ – }{\text{(aq)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{e}}^ – }\);
Negative/–/cathode
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \to {\text{Cu(s)}}\);
Ignore state symbols.
Award only [1] if electrodes not specified or if equations switched.
Observations:
blue colour of \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) fades;
Cu/metal deposited on negative/–/cathode/tin (jewellery);
gas produced/bubbles formed (at positive/+/anode);
pH of solution decreases/acidity increases (observed with indicator/pH paper);
Examiners report
Most candidates were able to place Ag, Cu and Pb in the correct order with the strongest reducing agent first although some equations given were not balanced. However, explanations were sometimes sloppy with statements such as “Pb is the strongest reducing agent because it reduces Cu and Ag” instead of \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{A}}{{\text{g}}^ + }\). Most candidates chose the correct oxidizing agent that could oxidize \({\text{C}}{{\text{l}}^ – }\) but not \({{\text{F}}^ – }\).
Electrolysis of molten sodium chloride was less well answered with incorrect use of states of matter and a lack of appreciation of their significance. In electrolysis of a concentrated solution of NaCl, many attempted to give explanations in terms of position in the electrochemical series without explaining the significance in terms of oxidising/reducing strength or the concentration of chloride ion. The half-equations taking place were not always correct. Sometimes the electrons were on the wrong side or the electrodes were reversed.
The part on electroplating was well answered although many did not pick up on the fact that, in this question, the anode is inert, leading to incorrect oxidation half-equation for the reaction at the positive anode. This question is an alert for teachers as it shows how easy candidates are taken by surprise by unexpected situations which are intrinsic to Aim 8.
