Question
X-ray crystallography of a metal crystal produces a diffraction pattern of bright spots.

Using X-rays of wavelength $1.54 \times 10^{-10} \mathrm{~m}$, the first bright spots were produced at an angle $\theta$ of $22.3^{\circ}$ from the centre.
Calculate the separation between planes of atoms in the lattice, in meters, using section 1 of the data booklet.
▶️Answer/Explanation
Markscheme
$$
\begin{aligned}
& « d=\frac{\mathrm{n} \lambda}{2 \sin \theta} » \\
& d=« \frac{1 \times 1.54 \times 10^{-10} \mathrm{~m}}{2 \times \sin 22.3^{\circ}} \rrbracket 2.03 \times 10^{-10} « \mathrm{~m} »
\end{aligned}
$$
Question
A feature of some \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra is the electron-withdrawing effect of electronegative atoms. These atoms cause nearby protons to produce peaks at higher chemical shift values, often in the range 2.5 to 4.5 ppm.
Consider the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, D, which has a molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) and is known to have an absorption in its IR spectrum corresponding to a C=O absorption.
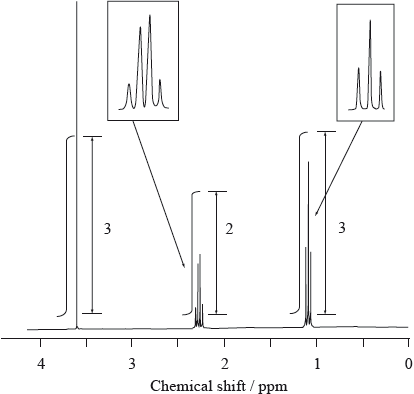
Use this information and the values in Table 18 of the Data Booklet to deduce the structure of D.
▶️Answer/Explanation
Markscheme
D could be \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\) or \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\);
this is because there are 3 peaks / 3:2:3 ratio;
explanation of splitting into a singlet a triplet and a quartet;
methyl propanoate/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\) is correct isomer because of higher chemical shift value of singlet (3.6 instead of 2.0–2.5);
Examiners report
From part (a) it is clear that proton NMR is poorly understood in some schools. Whilst many candidates could identify the correct structure, few could write a description of how they had obtained it from the information provided. Very few candidates explicitly mentioned that there were 3 peaks or explained how the structure had been determined, especially neglecting to explain the splitting patterns.
Question
Infrared spectroscopy is an analytical technique that uses electromagnetic radiation.
The infrared spectrum of a substance, X, with empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) is given below.
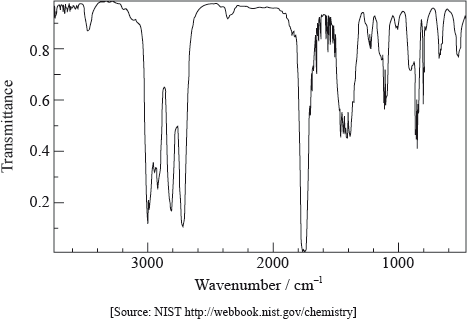
Predict the splitting pattern of the peak with the biggest area.
▶️Answer/Explanation
Markscheme
triplet/\(1:2:1\);
ECF if structure is incorrect.
Examiners report
Generally, once candidates had the structure correct, many had little trouble predicting the splitting pattern.
