Question
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) ![]() 2SO3 (g)
2SO3 (g)
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature. [3]
Answer/Explanation
Ans
pressure decrease «due to larger volume» ✓
reactant side has more moles/molecules «of gas» ✓
reaction shifts left/towards reactants ✓
Question
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
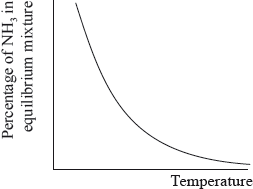
The percentage of ammonia in the equilibrium mixture varies with temperature.
Fertilizers may cause health problems for babies because nitrates can change into nitrites in water used for drinking.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Explain the effect of increasing the temperature on the rate of reaction.
(i) Define oxidation in terms of oxidation numbers.
(ii) Deduce the oxidation states of nitrogen in the nitrate, \({\text{NO}}_{\text{3}}^ – \), and nitrite, \({\text{NO}}_{\text{2}}^ – \), ions.
The nitrite ion is present in nitrous acid, HNO2, which is a weak acid. The nitrate ion is present in nitric acid, HNO3, which is a strong acid. Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
A small piece of magnesium ribbon is added to solutions of nitric and nitrous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since nitrous acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
The graph below shows how the conductivity of the two acids changes with concentration.
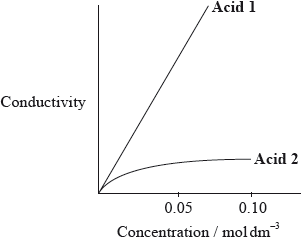
Identify Acid 1 and explain your choice.
Nitric acid reacts with silver in a redox reaction.
__ \({\text{Ag(s)}} + \) __ \({\text{NO}}_3^ – {\text{(aq)}} + \) ___ \( \to \) ___\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + \) __ \({\text{NO(g)}} + \) ____
Using oxidation numbers, deduce the complete balanced equation for the reaction showing all the reactants and products.
Answer/Explanation
Markscheme
(i) exothermic;
Accept either of the following for the second mark.
increasing temperature favours endothermic/reverse reaction;
as yield decreases with increasing temperature;
(ii) yield increases / equilibrium moves to the right / more ammonia;
increase in pressure favours the reaction which has fewer moles of gaseous products;
(iii) (rate increases because) increase in frequency (of collisions);
increase in energy (of collisions);
more colliding molecules with \(E \geqslant {E_{\text{a}}}\);
(i) increase in the oxidation number;
(ii) (NO3) + 5 and (NO2–) + 3;
Accept V and III.
Do not penalize missing charges on numbers.
strong acid completely dissociated/ionized and weak acid partially dissociated/ionized;
\({\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_3^ – {\text{(aq)}}\);
\({\text{HN}}{{\text{O}}_2}{\text{(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_2^ – {\text{(aq)}}\);
Allow only arrows as shown.
State symbols not needed.
Accept H2O and H3O+.
With HNO3:
faster rate of bubble/gas/hydrogen production;
faster rate of magnesium dissolving;
higher temperature change;
Accept opposite argument for HNO2.
Award [1] if 2 observations given but acid is not identified.
Reference to specific observations needed.
(i) (nitric acid) \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
(ii) not valid as nitrous acid reacts with same volume/ \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
HNO3;
(higher conductivity for solutions with same concentration as) there are more ions in solution;
change in oxidation numbers: Ag from 0 to +1 and N from +5 to +2;
Do not penalise missing charges on numbers.
balanced equation: \({\text{3Ag}} + {\text{NO}}_3^ – + {\text{4}}{{\text{H}}^ + } \to {\text{3A}}{{\text{g}}^ + } + {\text{NO}} + {\text{2}}{{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and product;
Award [3] for correct balanced equation.
Ignore state symbols
Examiners report
This was the most popular question and it was well answered by the majority of candidates. The reaction was correctly described as exothermic and the reason for this explained correctly in most cases. Most candidates knew that yield would increase with increased pressure, but failed to score a second mark because they did not mention ‘gaseous’ although they did know the answer. The effect of increased temperature on rate was generally well described although some did get confused with yield and how it would affect equilibrium.
Most candidates correctly defined oxidation in 6(b)(i) but ‘hedged their bets’ by stating loss of electrons as well as an increase in oxidation number. In 6(b)(ii) the oxidation states were generally deduced correctly but sometimes written as ionic charges (5+ for instance, instead of +5).
In 6(c) most correctly defined strong and weak acids, and many also wrote correct equations. A few, though, had no idea. In (c), arrows proved to be a minefield for several candidates, especially the equilibrium sign. HA was commonly given, as were CH3COOH and HCl, instead of nitric and nitrous acid.
6(d) presented problems with many candidates unable to describe observations and instead stating there would be ‘more hydrogen produced’ or just that ‘the reaction would be faster’. However, better candidates were able to answer this part correctly and scored full marks.
In 6(e)(i) the calculation was answered well, but 6(e)(ii), that asked for a comment on the hypothesis, was not and few candidates stated that the same volume of acid was needed.
In 6(f), the majority correctly identified the strong acid but often failed to explain its better conductivity in terms of the ions.
Many could give a correct balanced equation and scored the 3 marks, and others scored 1 mark for giving the correct reactants and products. However, not many candidates used oxidation numbers to deduce the balanced equation.
