Question
The buffer formed by carbon dioxide, $\mathrm{CO}_2(\mathrm{aq})$ and hydrogen carbonate ion, $\mathrm{HCO}_3{ }^{-}(\mathrm{aq})$, plays an important role in maintaining the $\mathrm{pH}$ of blood.
a. Calculate the $\mathrm{pH}$ of the buffer from the following data and section 1 of the data booklet.
$$
\begin{aligned}
& \mathrm{pK}_{\mathrm{a}}\left(\mathrm{CO}_2\right)=6.34 \\
& {\left[\mathrm{HCO}_3{ }^{-}(\mathrm{aq})\right]=1.40 \times 10^{-2} \mathrm{~mol} \mathrm{dm}^{-3}} \\
& {\left[\mathrm{CO}_2(\mathrm{aq})\right]=1.25 \times 10^{-3} \mathrm{~mol} \mathrm{dm}^{-3}}
\end{aligned}
$$
b. Explain the effect of a large amount of aspirin on the $\mathrm{pH}$ of blood.
▶️Answer/Explanation
Markscheme
a.
$$
« \mathrm{pH}=\mathrm{p} K_{\mathrm{a}}+\log _{10}\left(\frac{\left[\mathrm{HCO}_3^{-}\right]}{\left[\mathrm{CO}_2\right]}\right)=6.34+\log _{10}(11.2)=6.34+1.05 »=7.39
$$
[1 mark]
b. $\mathrm{H}^{+}$from aspirin reacts with $\mathrm{HCO}_3{ }^{-}$to form $\mathrm{CO}_2$ and $\mathrm{H}_2 \mathrm{O}$
OR
$$
\mathrm{H}^{+}(\mathrm{aq})+\mathrm{HCO}_3^{-}(\mathrm{aq}) \rightleftharpoons \mathrm{CO}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})
$$
OR
reverse reaction favoured «to use up some of the $\mathrm{H}^{+}$added»
$\mathrm{pH}$ decreases
No mark for “stating aspirin is a weak acid that dissociates partially to produce $\mathrm{H}^{+}$” without reference to reaction with $\mathrm{HCO}_3{ }^{-}$or to the equation.
Reversible arrows not required for the mark.
Do not accept “small pH change when small amount of $\mathrm{H}^{+}$is added”.
Question 2020
An investigation was carried out to determine the effect of chain length of the alcohol on the equilibrium constant, $K_{\mathrm{c}}$, for the reversible reaction:
$$
\mathrm{ROH}+\mathrm{CH}_3 \mathrm{COOH} \stackrel{\mathrm{H}^{+}(\mathrm{aq})}{\rightleftharpoons} \mathrm{CH}_3 \mathrm{COOR}+\mathrm{H}_2 \mathrm{O}
$$
The reactants, products and the catalyst form a homogeneous mixture.
Fixed volumes of each alcohol, the ethanoic acid and the sulfuric acid catalyst were placed in sealed conical flasks.
At equilibrium, the flasks were placed in an ice bath, and samples of each flask titrated with $\mathrm{NaOH}(\mathrm{aq}$ ) to determine the ethanoic acid concentration present in the equilibrium mixture.
The following processed results were obtained.
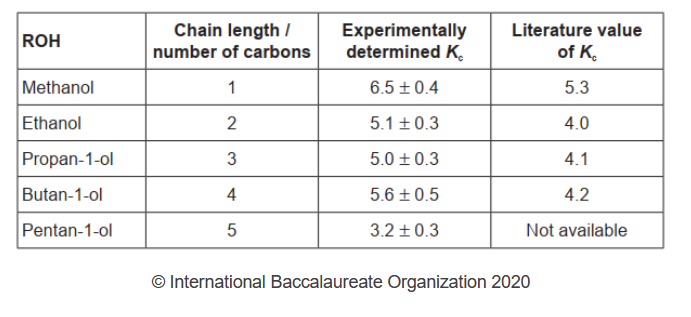
a.Identify the independent and dependent variables in this experiment.
b. The ice bath is used at equilibrium to slow down the forward and reverse reactions. Explain why adding a large amount of water to the reaction mixture would also slow down both reactions.
c. Suggest why the titration must be conducted quickly even though a low temperature is maintained.
d. An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with $\mathrm{NaOH}$ (aq). Outline why this experiment was necessary.
e. Calculate the percentage uncertainty and percentage error in the experimentally determined value of $K_{\mathrm{c}}$ for methanol.

f. Comment on the magnitudes of random and systematic errors in this experiment using the answers in (e).
g. Suggest a risk of using sulfuric acid as the catalyst.
▶️Answer/Explanation
Markscheme
a. Independent variable:
chain length $O R$ number of carbon «atoms in alcohol»
AND
Dependent variable:
volume of $\mathrm{NaOH} O R K_c$ lequilibrium constant $O R$ equilibrium concentration/moles of $\mathrm{CH}_3 \mathrm{COOH}$
b. dilution/lower concentrations
less frequent collisions «per unit volume»
Accept “Iowers concentration of acid catalyst” for M1. M2 must refer to increase in activation energy or different pathway.
Do not accept responses referring to equilibrium.
c. equilibrium shifts to left
OR
more ethanoic acid is produced «as ethanoic acid is neutralized»
OR
prevents/slows down ester hydrolysis
Accept “prevents equilibrium shift” if described correctly without direction.
d. to determine volume/moles of $\mathrm{NaOH}$ used up by the catalyst/sulfuric acid «in the titration»
OR
to eliminate/reduce «systematic» error caused by acid catalyst
Do not accept “control” OR “standard” alone.
e. Percentage uncertainty:
$$
« \frac{0.4 \times 100}{6.5}=» 6 \ll \% »
$$
Percentage error:
$$
« \frac{6.5-5.3}{5.3}=» 23 \ll \% »
$$
Award [1 max] if calculations are reversed OR if incorrect alcohol is used.
f. Any two:
large percentage error means large systematic error «in procedure» small percentage uncertainty means small random errors random errors smaller than systematic error
Award [2] for “both random and systematic errors are significant.”
g. corrosive/burns/irritant/strong oxidizing agent/carcinogenic OR disposal is an environmental issue OR causes other side reactions/dehydration/decomposition
Do not accept just “risk of accidents” OR “health risks” OR “hazardous”.
Question
In order to provide safe drinking water, a water supply is often treated with disinfectants, which aim to inactivate disease-causing bacteria in the water.
To compare the effectiveness of different disinfectants, a CT value is used as a measure of the dosage of disinfectant needed to achieve a certain level of inactivation of specific bacteria.
CT value (mg min dm−3) = C (mg dm−3) concentration of disinfectant × T (min) contact time with water
The table below compares the CT values of different disinfectants necessary to achieve 99% inactivation of two types of bacteria, listed as A and B.
(i) Deduce the oxidation state of chlorine in the following disinfectants.
(ii) From the data on CT values, justify the statement that bacterium B is generally more resistant to disinfection than bacterium A.
(iii) CT values can be used to determine whether a particular treatment process is adequate. Calculate the CT value, in mg min dm−3, when 1.50 × 10−5 g dm−3 of chlorine dioxide is added to a water supply with a contact time of 9.82 minutes.
(iv) From your answer to (a) (iii) and the data in the table, comment on whether this treatment will be sufficient to inactivate 99% of bacterium A.
CT values are influenced by temperature and by pH. The table below shows the CT values for chlorine needed to achieve 99% inactivation of a specific bacterium at stated values of pH and temperature.
(i) With reference to the temperature data in the table, suggest why it may be more difficult to treat water effectively with chlorine in cold climates.
(ii) Sketch a graph on the axes below to show how the CT value (at any temperature) varies with pH.
(iii) Comment on the relative CT values at pH 6.0 and pH 9.0 at each temperature.
(iv) Chlorine reacts with water as follows:
Cl2 (g) + H2O (l) \( \rightleftharpoons \) HOCl (aq) + HCl (aq)
HOCl (aq) \( \rightleftharpoons \) OCl− (aq) + H+ (aq)
Predict how the concentrations of each of the species HOCl (aq) and OCl− (aq) will change if the pH of the disinfected water increases.
Despite widespread improvements in the provision of safe drinking water, the sale of bottled water has increased dramatically in recent years. State one problem caused by this trend.
▶️Answer/Explanation
Markscheme
i
HOCl: +1
AND
ClO2: +4
Accept “I” and “IV” but not “1+/1” and “4+/4” notations.
ii
«most» CT values are higher for «bacterium» B
OR
«generally» higher dosage needed for «bacterium» B
Accept converse arguments. Accept “concentration” for “dosage”
iii
«CT = 1.50 × 10–5 × 103 mg dm–3 × 9.82 min =» 1.47 × 10–1 «mg min dm–3»
iv
lower than CT value/minimum dosage/1.8 × 10–1 «mg min dm–3»
AND
no/insufficient
Accept “concentration” for “dosage”.
i
higher CT value at lower temperature
OR
higher dosage «of chlorine» needed at low temperature
Accept “effectiveness decreases at lower temperature”.
Accept “concentration” for “dosage”.
Accept converse arguments.
ii
labeled axes ( y: CT and x: pH)
AND
curve with increasing gradient
Do not accept axes the wrong way round.
Accept a linear sketch.
iii
values at pH 9.0 approximately 3 times values at pH 6.0
OR
increase in CT values in same ratio
The exact ratio is 2.9 times
Do not accept just “increase in value”.
iv
[HOCl] decreases AND [OCl−] increases
plastic disposal/pollution
OR
plastic bottles use up petroleum/non-renewable raw material
OR
chemicals in plastic bottle can contaminate water
OR
«prolonged» storage in plastic can cause contamination of water
OR
plastic water bottles sometimes reused without proper hygiene considerations
Accept other valid answers.
Accept economic considerations such as “greater production costs”, “greater transport costs” or “bottled water more expensive than tap water”
Question
A link between the combustion of fossil fuels and an increase in the temperature of the Earth’s atmosphere was proposed over a century ago.
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
▶️Answer/Explanation
Markscheme
computers can now carry out more complex calculations
OR
better understanding of the interactions between the various systems involved
OR
clear evidence of global warming
OR
«reliable» global temperature data now available
OR
techniques have been available to monitor carbon dioxide levels
Accept “better/faster computers”.
Accept “better modelling”.
Accept “better/more reliable/consistent data”.
Accept “better measuring techniques”.
Accept other scientifically based (not politically based) reasons.
Accept if specific relevant data is given.
Do not accept “increased combustion of fossil fuels” or “increased concerns about global warming”.
[1 mark]
symmetric stretching will not absorb IR
OR
asymmetric stretching will absorb IR
change in polarity/dipole «moment» required «to absorb IR»
[2 marks]
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq) «and pH decreases»
OR
CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq) AND H2CO3(aq) \( \rightleftharpoons \) H+(aq) + HCO3–(aq) «and pH decreases»
Accept reversible or non-reversible arrows for all.
[1 mark]
reduce it AND absorbing/reflecting sunlight
Accept “reduce it because of global dimming”.
Accept “reduce it AND blocking sunlight”.
[1 mark]
Question
The buffer formed by carbon dioxide, CO2(aq) and hydrogen carbonate ion, HCO3−(aq), plays an important role in maintaining the pH of blood.
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 mol\(\,\)dm−3
[CO2(aq)] = 1.25 × 10−3 mol\(\,\)dm−3
Explain the effect of a large amount of aspirin on the pH of blood.
▶️Answer/Explanation
Markscheme
«pH = pKa + log10 \(\left( {\frac{{\left[ {{\text{HC}}{{\text{O}}_3}^ – } \right]}}{{\left[ {{\text{C}}{{\text{O}}_2}} \right]}}} \right) = 6.34 + {\log _{10\;}}(11.2) = 6.34 + 1.05\)» = 7.39
[1 mark]
H+ from aspirin reacts with HCO3− to form CO2 and H2O
OR
H+(aq) + HCO3−(aq) \( \rightleftharpoons \) CO2(aq) + H2O(l)
OR
reverse reaction favoured «to use up some of the H+ added»
pH decreases
No mark for “stating aspirin is a weak acid that dissociates partially to produce H+” without reference to reaction with HCO3− or to the equation.
Reversible arrows not required for the mark.
Do not accept “small pH change when small amount of H+ is added”.
[2 marks]
Question
There is a link between world energy consumption and carbon dioxide production.
Climate induced changes in the ocean can be studied using measurements such as the Atmospheric Potential Oxygen (APO). Trends in APO concentration from two stations, one in each hemisphere, are shown below.
Trends in atmospheric potential oxygen (APO) based on monthly averages between 1990 and 2010.
[Source: www.ioos.noaa.gov]
The following graph represents world energy consumption by type for the years 1988–2013.
Estimate the percentage of energy consumption which did not directly produce CO2 in 2013.
O2 is consumed in producing CO2 for electricity generation. The graph shows the relationship between the world’s electricity generation and CO2 production between 1994 and 2013.
Calculate the mass, in million tonnes, of oxygen gas ultimately found in CO2 which is consumed in generating 18\(\,\)000 terawatts of electricity using the equation given for the best fit line. Give your answer to 2 significant figures.
Assume coal is the only energy source.
The equilibrium expression for O2 exchange between the atmosphere and ocean is O2(g) \( \rightleftharpoons \) O2(aq). Identify one factor which shifts the equilibrium to the right.
Factors such as photosynthesis and respiration are excluded so that APO is influenced by oceanic changes only. Suggest why the seasonal cycles from Alert station and Cape Grim observatory are different.
The change in APO O2/N2 ratio, per meg, is measured relative to an O2/N2 reference.
\[\Delta {\text{(}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{N}}_{\text{2}}}{\text{)}} = \left( {\frac{{{{({{\text{O}}_2}/{{\text{N}}_2})}_{{\text{sample}}}}}}{{{{({{\text{O}}_2}/{{\text{N}}_2})}_{{\text{reference}}}}}} – 1} \right) \times {10^6}\]
Calculate the APO Δ(O2/N2) value for an oxygen concentration of 209\(\,\)400 ppm assuming that any change in N2 concentration is negligible. Reference values for O2 and N2 are 209 460 and 790 190 ppm respectively.
Suggest a reason for the general negative gradient of the APO curve given in (c).
▶️Answer/Explanation
Markscheme
«\(\frac{{\sum {({\text{renewables}} + {\text{hydroelectricity}} + {\text{nuclear)}}} }}{{{\text{total}}}}\)»
«\(\left( {\frac{{8800 – 7200}}{{12600}}} \right) \times 100 = \)» 13 «%»
Accept range of “11–16%”.
[1 mark]
«18000 = 0.54x – 2000»
x = 37037 «million tonnes of CO2»
«\(\frac{{32.00}}{{44.01}}\) x 37037 = 26930»
27000/2.7 x 104 «million tonnes of O2»
Accept “37000 «million tonnes of CO2»” for M1.
Award [2] for correct final answer with two significant figures.
Award [1] for non rounded answers in range 26903–26936 «million tonnes of O2».
[2 marks]
increase in «atmospheric» pressure
OR
increase in [O2(g)]/concentration of O2(g)
OR
decrease in [O2(aq)]/concentration of O2(aq)
OR
decrease in temperature
Accept “increase in volume of oceans «due to polar ice cap melting»” OR “consumption of O2 in oceans/O2(aq)
«by living organisms»”.
State symbols required for oxygen concentration.
[1 mark]
summer in one station while winter in other
OR
stations are at different latitudes
oxygen dissolves better in colder water
Accept “opposite seasons «in each hemisphere»”.
Do not accept “different locations with different temperatures” OR “stations are in different hemispheres”.
[2 marks]
«\({\text{(}}\frac{{209400}}{{209460}} – 1{\text{)}} \times {10^6}\) =» − 286.5 «per meg»
The nitrogen cancels so is not needed in the calculation.
Negative sign required for mark.
[1 mark]
decrease in [O2]/concentration of O2
OR
increasing combustion of fossil fuels «consumes more O2 so [O2]/concentration of O2 decreases»
OR
warmer oceans/seas/water «as oxygen dissolves better in colder water»
OR
deforestation
Accept “decrease in level of O2”.
Accept “increasing CO2 production «consumes more O2 so [O2]/concentration of O2 decreases»”.
Do not accept “decrease in amount of O2” OR “increase in greenhouse gases”.
[1 mark]
Question
Disposable plastic lighters contain butane gas. In order to determine the molar mass of butane, the gas can be collected over water as illustrated below:
List the data the student would need to collect in this experiment.
Explain why this experiment might give a low result for the molar mass of butane.
Suggest one improvement to the investigation.
▶️Answer/Explanation
Markscheme
mass/m of lighter before AND after the experiment
volume of gas/Vgas «collected in the cylinder»
«ambient» pressure/P «of the room»
temperature/T
Accept “change in mass of lighter”.
Accept “weight” for “mass”.
Do not accept just “mass of lighter/gas”.
Accept “volume of water displaced”.
Do not accept “amount” for “volume” or “mass”.
[4 marks]
Any two of:
pressure of gas not equalized with atmospheric/room pressure
too large a recorded volume «of gas produces a lower value for molar mass of butane»
OR
cylinder tilted
difficult to dry lighter «after experiment»
OR
higher mass of lighter due to moisture
OR
smaller change in mass but same volume «produces lower value for molar mass of butane»
using degrees Celcius/°C instead of Kelvin/K for temperature
Accept “vapour pressure of water not accounted for” OR “incorrect vapour pressure of water used” OR “air bubbles trapped in cylinder”. Do not accept “gas/bubbles escaping «the cylinder»” or other results leading to a larger molar mass.
Accept “lighter might contain mixture of propane and butane”.
Do not accept only “human errors” OR “faulty equipment” (without a clear explanation given for each) or “mistakes in calculations”.
[2 marks]
record vapour pressure of water «at that temperature»
OR
equalize pressure of gas in cylinder with atmospheric/room pressure
OR
tap cylinder before experiment «to dislodge trapped air»
OR
collect gas using a «gas» syringe/eudiometer/narrower/more precise graduated tube
OR
collect gas through tubing «so lighter does not get wet»
OR
dry lighter «before and after experiment»
OR
hold «measuring» cylinder vertical
OR
commence experiment with cylinder filled with water
Accept “adjust cylinder «up or down» to ensure water level inside cylinder matches level outside”.
Accept “repeat experiment/readings «to eliminate random errors»”.
Accept “use pure butane gas”.
[1 mark]
Question
The combustion of fossil fuels produces large amounts of CO2, a greenhouse gas.
The diagram below illustrates a range of wavelengths in the electromagnetic spectrum.
Synthesis gas, or syngas, mainly composed of CO(g) and H2(g), is an alternative form of fuel. It can be produced by coal or biomass gasification, passing steam over the source material in a low oxygen environment.
Identify which region, A or B, corresponds to each type of radiation by completing the table.
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) \( \rightleftharpoons \) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
Suggest an equation for the production of syngas from coal.
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
Suggest a reason why syngas may be considered a viable alternative to crude oil.
▶️Answer/Explanation
Markscheme
Accept “B” alone for incoming radiation from sun.
All three correct answers necessary for mark.
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)
State symbols AND equilibrium arrow required for mark.
Accept
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq).
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq).
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
OR
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + H2O(l) \( \rightleftharpoons \) H3O+(aq) + HCO3–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + 2H2O(l) \( \rightleftharpoons \) 2H3O+(aq) + CO32–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
equilibrium shifts to the right causing increase in [H3O+]/[H+ ] «thereby decreasing pH»
Equilibrium sign needed in (b) (ii) but penalize missing equilibrium sign once only in b (i) and (ii).
Do not accept “CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)” unless equation was not given in b (i).
[2 marks]
C(s) + H2O(g) → CO(g) + H2(g)
OR
3C(s) + H2O(g) + O2(g) → 3CO(g) + H2(g)
OR
4C(s) + 2H2O(g) + O2(g) → 4CO(g) + 2H2(g)
OR
5C(s) + H2O(g) + 2O2(g) → 5CO(g) + H2(g)
Accept other correctly balanced equations which produce both CO AND H2.
[1 mark]
8CO(g) + 17H2(g) → C8H18(l) + 8H2O(g)
[1 mark]
coal more plentiful than crude oil
OR
syngas can be produced from biomass/renewable source
OR
syngas can undergo liquefaction to form octanes/no need to transport crude
OR
syngas can be produced by gasification underground, using carbon
OR
capture/storage «to not release CO2 to the atmosphere»
OR
coal gasification produces other usable products/slag
[1 mark]

