Question
Most metals have to be extracted from an ore. The way in which this is carried out depends on the reactivity of the metal.
(a) Identify a metal produced by reacting its oxide with carbon or carbon monoxide.
Use section 25 of the data booklet.
(b) Aluminium is produced by electrolytic reduction of a solution of aluminium oxide, \(Al_2O_3\), in molten cryolite, \(Na_3AlF_6\).
(i) Write the half equation for the reaction at the electrode where aluminium is formed.
(ii) Calculate the atom economy for the production of aluminium from its oxide, assuming the products do not react with the electrodes. Use section 1 of the data booklet.
(iii) Suggest one factor, other than atom economy, that indicates the production of aluminium from its ore has a significant environmental impact.
(iv) Deduce why pure molten aluminium oxide is a poor conductor of electricity.
Use sections 8 and 29 of the data booklet.
(c) Inductively coupled plasma (ICP) techniques can be used to estimate the concentration of other metals in the aluminium produced.
(i) Describe the plasma state.
(ii) Explain how different metals are identified, and their concentrations determined, if ICP is coupled with Optical Emission Spectroscopy (OES).
Identification:
Concerntration:
(d) An aluminium matrix can be reinforced with carbon nanotubes. Outline why carbon nanotubes are so strong and rigid.
Answer/Explanation
Answer:
(a) Any one of:
Zn, Cr, Fe, Cd, Co, Ni, Sn, Pb, Sb, As, Bi, Cu, Ag, Pd, Hg, Pt
(b) (i) \(Al^{3+} + 3 e^-\) → Al(l)
(ii) 〈〈\(\frac{2 \times 26.98}{2 \times 26.98 + 3 \times 16.00} \times 100\) = 〉〉 52.92%
(iii) high energy consumption «that has environmental implications»
OR
large amounts of waste «produced by mining and purification of the ore»
OR
mining has negative impact on landscape
OR
greenhouse gas/pollution from transport/machinery
(iv) average electronegativity 2.5 AND
electronegativity difference 1.8
border between ionic and «polar» covalent
(c) (i) electrons AND «positive» ions «in gaseous state»
(ii) Identification:
«emit» light/photons of characteristic frequencies
Concentration:
intensity/brightness of light «proportional to concentration»
(d) «held together by strong» covalent bonds «and defect free/regular 2D/3D»
Question
Metals are often alloyed for desired characteristics.
(a) Explain why metals alloyed with another metal are usually harder and stronger but poorer conductors than the pure metal.
(b) Bronze is one of the oldest known alloys. Inductively coupled plasma spectroscopy can be combined with mass spectrometry (ICP–MS) or optical emission spectroscopy (ICP–OES) to analyse the structure and composition of alloys.
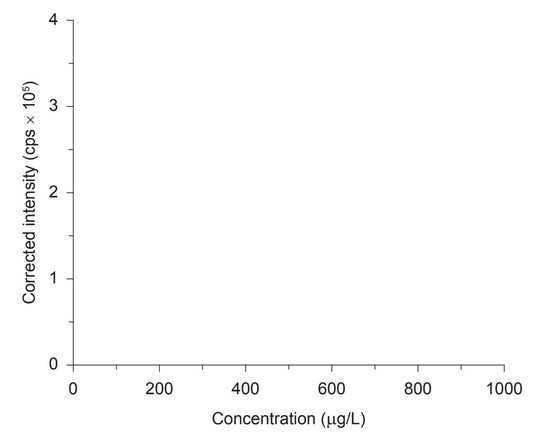
Sketch a typical calibration curve for ICP–OES.
(c) Various bronze shields from Ayanis fortress in Turkey, dated 670 bce, were analysed using ICP–MS.
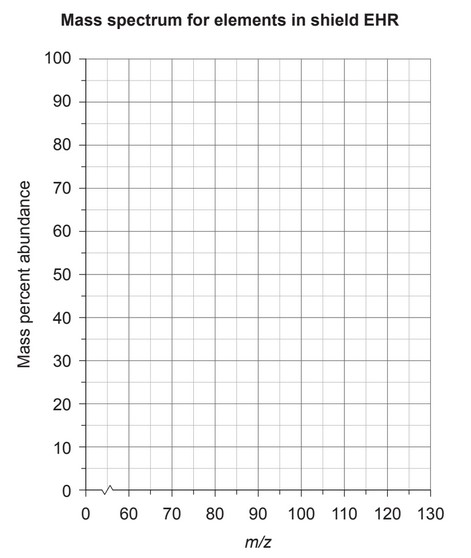
(i) Draw the expected mass spectrum produced by shield EHR for the range given on the graph. Use relative atomic mass, ignoring isotopes.
(ii) Explain the role of the inductively coupled plasma (ICP) torch in allowing the sample to be injected into the mass spectrometer for analysis.
Answer/Explanation
Answer:
(a) metal ions/atoms have different sizes
cations/atoms/layers do not slide over each other as easily
«irregularities» obstruct free movement of electrons
(b) straight line increasing and going through origin/(0,0)
(c) (i) 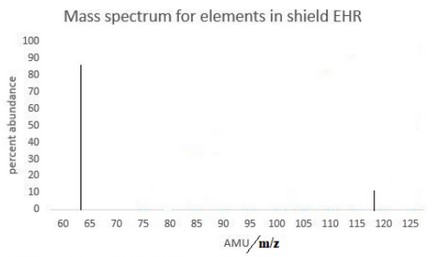
89% at 63.5 AND 10% at 118
(c) (ii) vapourize «the sample»
ionize «the sample»
