Question
Polybutadiene, used in truck tyres, is a polymer of buta-1,3-diene. The spatial arrangement of atoms in the polymer depends on the type of catalyst used.
a. Outline two differences between heterogeneous and homogeneous catalysts.
b. Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
c(i).Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”. c(ii)The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
▶️Answer/Explanation
Markscheme
a. Any two of:
heterogeneous catalyst is in different phase than reactants AND homogeneous catalyst in same phase [ $\boldsymbol{V}$ ]
homogeneous catalysts chemically change/react and are reformed at end of reaction
OR
reactants adsorb onto heterogenous catalyst and products desorb [ $\checkmark$ ]
heterogeneous catalysts are more easily removed than homogenous catalysts $[\boldsymbol{V}]$
heterogeneous catalysts can function at higher temperatures $[\boldsymbol{V}]$
homogeneous catalysts are «generally» more selective $[\boldsymbol{}]$
homogeneous catalysts offer a broader range of reactions $[\boldsymbol{\sim}]$
Note: Accept “state” for “phase”.
Accept “heterogeneous catalyst provides a surface to activate reaction”.
b. elastomers bend under force «and return to original form when force is released»
OR
elastomers make tyre more flexible $[\boldsymbol{}]$
allows greater contact with road $[\boldsymbol{}]$
c(i)does not contain heterocyclic ring with 2 oxygen atoms
OR
middle ring has only 1 oxygen atom $[\boldsymbol{V}]$
produces similar toxic effects to dioxins $[\boldsymbol{V}]$
Note: Accept “does not contain dioxin ring” for M1.
c(ii)taken up by plants, which are eaten by animals «and then further dispersed»
OR
passed on in food chain $[\boldsymbol{U}]$
Note: Accept “do not break down and can be remobilised as dust”.
Question
Metals are extracted from their ores by several methods, including electrolysis and reduction with carbon.
a. Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of $48250 \mathrm{C}$. Use sections 2 and 6 of the data booklet.
b. Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
c. Explain the action of metals as heterogeneous catalysts.
d. Outline how alloys conduct electricity and why they are often harder than pure metals.
Conduct electricity:
Harder than pure metals:
e. Carbon nanotubes are added to metals to increase tensile strength.
Write an equation for the formation of carbon nanotubes from carbon monoxide.
▶️Answer/Explanation
Markscheme
a. moles of electrons $«=\frac{48250 \mathrm{C}}{96500 \mathrm{C} \mathrm{mol}^{-1}} »=0.5000$ «mol»[ $\left.\checkmark\right]$
moles of aluminium $«=\frac{0.5000 \mathrm{~mol}}{3} »=0.1667$ «ol» [ $]$
mass of aluminium $«=26.98 \mathrm{~g} \mathrm{~mol}^{-1} \times 0.1667 \mathrm{~mol} »=4.50 « \mathrm{~g} »[V]$
Note: Award [3] for correct final answer.
b. Any two of:
larger linear calibration [ $\boldsymbol{V}]$
«accurate» detection of multiple elements/metals $[\boldsymbol{\sim}]$
«accurate» detection of elements in low concentration $[\boldsymbol{V}]$
temperature around $10000 \mathrm{~K}$ atomises/ionises every material $[\boldsymbol{}]$
c. Any two of:
reactant(s) adsorb onto active sites/surface [ $\boldsymbol{V}]$
bonds weakened/broken/stretched «in adsorbed reactants»
OR
activation energy lowered $[\boldsymbol{V}]$
products desorbed [ $\boldsymbol{V}]$
Note: Accept “products released” for M3.
d. Conduct electricity:
«delocalized/valence» electrons free to move «under potential difference» [ $\boldsymbol{C}]$
Harder than pure metals:
atoms/ions of different sizes prevent layers «of atoms/ions» from sliding over one another [ $\boldsymbol{V}]$
e. $2 \mathrm{CO}(\mathrm{g}) \rightarrow \mathrm{C}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})[\boldsymbol{}]$
Question
Catalysts are commonly used in industry.
a. Describe how a heterogeneous catalyst provides an alternative pathway for a reaction.
b. Distinguish between heterogeneous and homogeneous catalysts, giving one difference.
c. Nanotubes are used to support the active material in nanocatalysts.
Explain why oxygen cannot be used for the chemical vapour deposition (CVD) preparation of carbon nanotubes.
▶️Answer/Explanation
Markscheme
a. reactant(s) adsorb onto active sites/surface
NOTE: Do not accept “absorb” for “adsorb” for M1.
Accept “bonds to” for “adsorb” for M1.
«reactant» bonds weakens «and products are desorbed»
NOTE: Accept “bonds break/stretch “and products are desorbed»” for M2.
Award [1 max] for “lowers activation energy”.
b. Any one of the following:
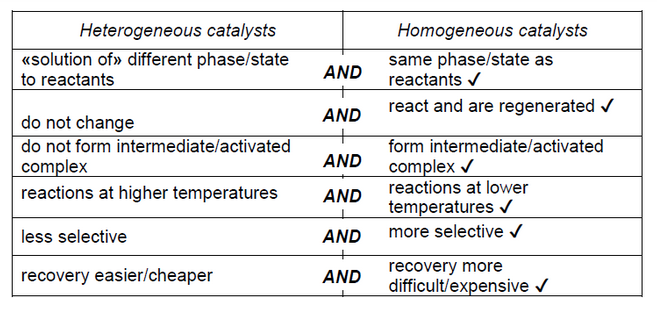
NOTE: Accept “heterogeneous adsorb reactants and homogeneous” but do not accept “absorb” for “adsorb”.
Accept “heterogeneous have active sites and homogeneous do not”.
c. high temperature used
oxygen $/ \mathrm{O}_2$ reacts with carbon/C
OR
carbon dioxide/ $\mathrm{CO}_2$ can form
Question
One way of classifying materials is based on the type of bonding present.
One reaction to convert cyclohexanone to caprolactam using concentrated sulfuric acid as a catalyst is shown.
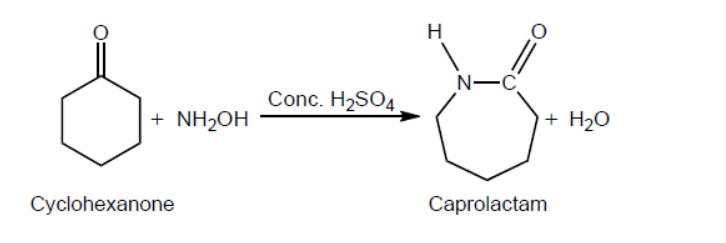
a. Outline why this type of classification is not entirely satisfactory by using magnesium diboride, $\mathrm{MgB}_2$, as an example. Refer to sections 8 and 29 of the data booklet.
b.i. Structures of poly(methyl acrylate), PMA, and Bakelite ${ }^{\circledR}$ are shown.
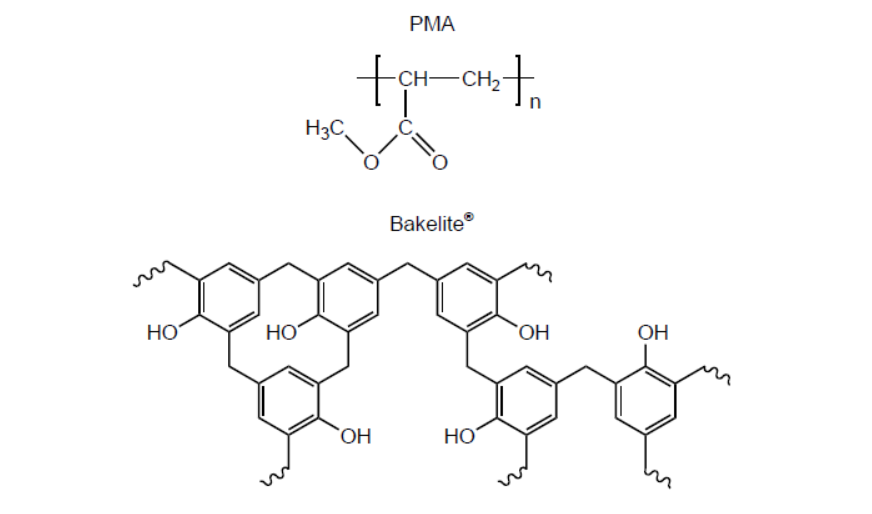
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
b.ii.In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
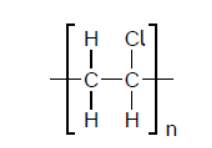
Formulate an equation for this reaction using the formula of the PVC repeating unit.
c.i. A zeolite is an alternative catalyst for this reaction.
Explain how zeolites act as selective catalysts.
c.ii.Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
d. Repeating units of several polymers are listed.
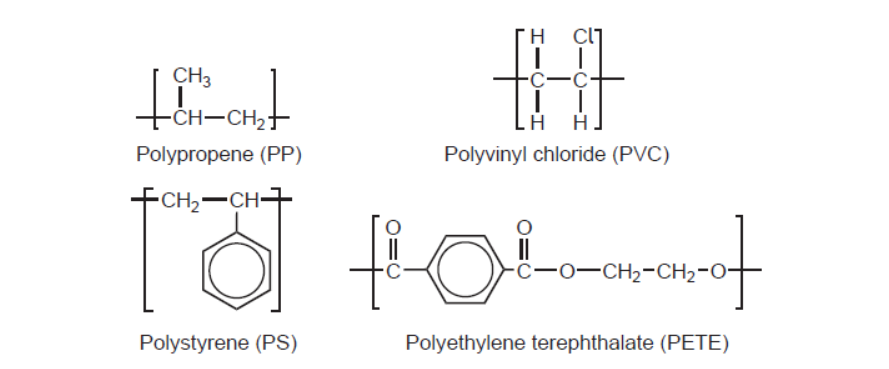
The infrared (IR) spectrum of one of these polymers is shown.
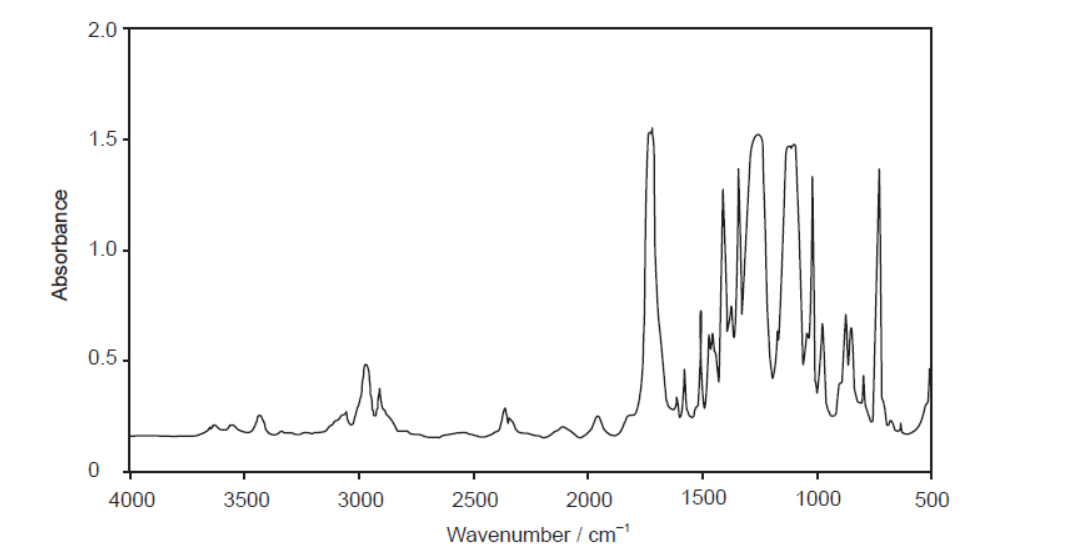
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
▶️Answer/Explanation
Markscheme
a. $\Delta \chi=0.7$ AND average $\Delta \chi=1.7$
bonding between metallic and ionic
OR
more than one type of bonding present
OR
bond type difficult to determine as close to several regions/several types/named bonding types «eg ionic and covalent etc.»
OR
bond is mostly covalent «based on \% covalent scale on diagram»
OR
bond has « $\frac{0.7}{3.2} \times 100=» 22 \%$ ionic character
Accept “EN” for ” $\chi$ “.
Accept “bond is ionic but close to several regions/several types/other named bonding type(s) (eg covalent, metallic and covalent etc.)”.
Do not accept just “bond is ionic”.
Accept any value for $\%$ ionic character in range $15-24 \%$ or $\%$ covalent character in range $76-85 \%$.
b.i. Thermoplastic polymer:
PMA AND «weak» intermolecular/IMFs/London/dispersion/van der Walls/vdW/dipole-dipole forces «between layers/chains» OR
PMA AND no/few cross-links «between layers/chains»
Thermosetting polymer:
Bakelite ${ }^{\circledR}$ AND «strong» covalent bonds «between layers/chains»
OR
Bakelite ${ }^{\circledR}$ AND extensive cross-links «between layers/chains»
Do not accept “hydrogen bonding” for M1.
Award [1 max] for correct reasons for both polymer classes even if named polymers are incorrectly classified.
b.ii. $\mathrm{CH}_2 \mathrm{CHCl}(\mathrm{s})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{HCl}(\mathrm{g})+\mathrm{CO}(\mathrm{g})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})$
OR
$\mathrm{CH}_2 \mathrm{CHCl}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{HCl}(\mathrm{g})+2 \mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})$ AND $2 \mathrm{CO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})$
Accept any correctly balanced equation that includes the products specified.
c.i. pores/cavities/channels/holes/cage-like structures «in zeolites» have specific shape/size
only reactants «with appropriate size/geometry» fit inside/go through/are activated/can react
c.ii.does not require corrosive acid/«concentrated» sulfuric acid/ $\mathrm{H}_2 \mathrm{SO}_4$
OR
zeolite can be recycled «more easily»
OR
product can be «more» easily separated from a zeolite «than from sulfuric acid»
OR
minimal/less impact on environment
OR
synthesis of specific isomers as products
d. Name and reason:
PET/PETE AND peak for $\mathrm{C}=\mathrm{O}$ «at $1700-1750 \mathrm{~cm}^{-1} »$
RIC:
1
Accept “PETIPETE AND peak for C-O «at 1050-1410 cm”-1»” for M1.
Accept “PET/PETE AND peak(s) for COO” for M1.
Accept name or abbreviation for polymer.
No ECF for $M 2$.
Question
Nano-sized ‘test-tubes’ with one open end, can be formed from carbon structures.
Carbon nanotubes can be used as catalysts.
Describe these ‘test-tubes’ with reference to the structures of carbon allotropes.
These tubes are believed to be stronger than steel. Explain the strength of these ‘test-tubes’ on a molecular level.
Suggest two reasons why they are effective heterogeneous catalysts.
State one potential concern associated with the use of carbon nanotubes.
▶️Answer/Explanation
Markscheme
walls have rolled/single sheets of graphite/carbons bonded in hexagons;
ends have half a buckyball (fullerene)/carbons in pentagons (and hexagons);
covalent bonds are very strong;
large surface area;
Do not accept “reactive surface”.
high selectivity related to dimensions of tube;
unknown health effects;
Accept potentially harmful as easily ingested/inhaled.
Accept difficulty of preparing nanotubes in required amounts.
Examiners report
Responses to this question were generally poor perhaps reflecting the unfamiliarity of candidates with the new syllabus content.
Responses to this question were generally poor perhaps reflecting the unfamiliarity of candidates with the new syllabus content.
Responses to this question were generally poor perhaps reflecting the unfamiliarity of candidates with the new syllabus content.
Responses to this question were generally poor perhaps reflecting the unfamiliarity of candidates with the new syllabus content.
Question
State the difference between homogeneous and heterogeneous catalysts.
State one advantage and one disadvantage that homogeneous catalysts have over heterogeneous catalysts.
Advantage:
Disadvantage:
Apart from their selectivity to form the required product and their cost, discuss two other factors which should be considered when choosing a suitable catalyst for an industrial process.
▶️Answer/Explanation
Markscheme
homogeneous catalysts are in the same phase/state as the reactants and heterogeneous catalysts are in a different phase/state to the reactants;
Advantage
often works faster / all the catalyst is exposed to the reactants / OWTTE;
Disadvantage
difficult to remove the catalyst from the products / more limited range of acceptable catalysts;
efficiency;
ability to work under a variety of conditions;
environmental impact;
problems with the catalyst becoming poisoned (by impurities);
Examiners report
Most candidates correctly stated the difference between homogeneous and heterogeneous catalysts.
Most candidates could state one advantage and one disadvantage of homogeneous catalysts.
Candidates were less successful in part (c) in discussing the choice of a suitable catalyst.
Question
Catalytic cracking uses heterogeneous catalysts.
The initial products of the fractional distillation of oil often undergo cracking. This can be carried out in a number of ways. State the major reason for choosing each of the following techniques.
Catalytic cracking:
Thermal cracking:
Steam cracking:
Explain how these differ from homogeneous catalysts.
Identify one disadvantage of using heterogeneous catalysts.
Many of the compounds produced by cracking are used in the manufacture of addition polymers. State the essential structural feature of these compounds and explain its importance.
The polymers often have other substances added to modify their properties. One group of additives are plasticizers. State how plasticizers modify the physical properties of polyvinyl chloride and explain at the molecular level how this is achieved.
▶️Answer/Explanation
Markscheme
Catalytic cracking:
used to produce moderate length alkanes (for fuels) / lower temperature / lower energy consumption / more control of product;
Thermal cracking:
used to crack very long chain starting material;
Steam cracking:
used to produce low molar mass alkenes (for petrochemicals);
heterogeneous catalysts in a different phase to the reactants / homogeneous catalysts in the same phase as reactants;
easily poisoned / efficiency decreases over time / forms clumps / only effective on surface / require high surface area;
carbon-carbon double bond;
breaks allowing addition reaction / allows monomers/molecules to join together/polymerize;
make the polymer more flexible;
fits between/increases separation between polymer chains / allow polymer chains to slide past each other more easily / weaken intermolecular attraction;
Examiners report
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well known
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well known
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well known
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well known
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well known
Question
Catalysts may be homogeneous or heterogeneous.
Distinguish between homogeneous and heterogeneous catalysts.
Discuss two factors which need to be considered when selecting a catalyst for a particular chemical process.
Identify the catalyst used in the catalytic cracking of long chain hydrocarbons and state one other condition needed.
State an equation for the catalytic cracking of the straight chain hydrocarbon pentadecane, \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{32}}}}\), to produce two products with similar masses.
▶️Answer/Explanation
Markscheme
homogeneous catalysts are in the same phase/state as reactants and heterogeneous catalysts are in a different phase/state to reactants;
should produce only the desired product / selectivity;
efficiency;
should be able to work under both mild and severe conditions / should be able to work at high temperatures;
should not produce an (unwanted) environmental impact;
cost / economic viability / OWTTE;
ease of poisoning/contamination;
(catalyst a mixture of) silica/silicon dioxide/ \({\text{Si}}{{\text{O}}_2}\) and alumina/aluminium oxide/\({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) / zeolites/aluminosilicates;
high temperature / 500 °C;
\({{\text{C}}_{15}}{{\text{H}}_{32}} \to {{\text{C}}_8}{{\text{H}}_{18}} + {{\text{C}}_7}{{\text{H}}_{14}}/{{\text{C}}_{15}}{{\text{H}}_{32}} \to {{\text{C}}_8}{{\text{H}}_{16}} + {{\text{C}}_7}{{\text{H}}_{16}}\);
Examiners report
Most candidates were aware of the differences between homogeneous and heterogeneous catalysts.
[N/A]
Few candidates could name the catalyst but knew one other condition needed for catalytic cracking.
Most correctly stated an equation for the catalytic cracking of pentadecane, but some added oxygen or water, and some had too many hydrogen atoms in the products.
Question
State three factors which need to be considered when an industrial catalyst is chosen. In each case explain why they are important.
▶️Answer/Explanation
Markscheme
This was related to assessment statement C.4.3. It proved to be challenging; candidates did not show a good understanding and answers were not well organized.
Examiners report
selectivity – does the catalyst give a good yield of the desired compound / OWTTE;
efficiency – how much faster does the reaction occur/reach equilibrium / OWTTE;
economic – cost / how long the catalyst will last / how easily is it poisoned / OWTTE;
environmental impact – toxicity / regeneration / recyclability/ease of separation of catalyst from reaction mixture / OWTTE;
versatility – ability to work under a range of conditions / ability to work with a range of substances / OWTTE;
Award [1 max] for any three correct factors without explanation.
Question
Cracking is the process by which long-chain alkanes found in oil are broken down into smaller molecules.
The following reaction occurs during the cracking of tetradecane, \({{\text{C}}_{{\text{14}}}}{{\text{H}}_{{\text{30}}}}\).
\[{{\text{C}}_{14}}{{\text{H}}_{30}}{\text{(g)}} \to {{\text{C}}_{10}}{{\text{H}}_{22}}{\text{(g)}} + {\text{2}}{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}}\]
Suggest a use for each of the products formed in the reaction.
\({{\text{C}}_{{\text{10}}}}{{\text{H}}_{{\text{22}}}}\):
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\):
State the main type of product obtained from steam cracking.
Catalytic cracking uses silica as a heterogeneous catalyst. Explain the mode of action of a heterogeneous catalyst.
State one advantage of using a heterogeneous catalyst rather than a homogeneous catalyst.
Discuss two factors that need to be considered when choosing a catalyst for a process.
▶️Answer/Explanation
Markscheme
C10H22: gasoline/petrol / fuel / kerosene;
Do not allow just combustion or cars.
Allow gas for cars/automobiles instead of gasoline but not gas alone.
C2H4: chemical feedstock / OWTTE;
Accept suitable example such as manufacturing plastics/polymers but not just plastics.
alkenes;
solid surface has active sites / reactants adsorb on solid surface;
Do not accept absorb instead of adsorb.
brings reactants close together in correct orientation;
weakens reactant bonds / reactants bonds are easier to break;
can be easily removed/filtered from reaction mixture / large amount of reactant molecules pass over catalyst that is in a fixed position / can be used at high temperatures;
selectivity to produce (a high yield of) the desired product / OWTTE;
extent to which rate of reaction is increased/Ea is lowered;
amount of reactant converted to product per amount of catalyst;
Accept efficiency / conversion rate.
ability to work under different/a range of conditions;
environmental/health impact;
catalytic poisoning / active sites become blocked;
cost in relation to life expectancy / OWTTE;
ease of removal from reaction mixture;
Examiners report
Less than half of the candidates knew the uses of the products of cracking in (a) and very few candidates knew the product of steam cracking in (b).
Less than half of the candidates knew the uses of the products of cracking in (a) and very few candidates knew the product of steam cracking in (b).
The mode of action of heterogeneous catalysts was also not well answered. The majority of candidates wrote about catalysts in general gaining no marks on part (c).
Parts (d) and (e) about the advantage of heterogeneous catalysts over homogeneous catalysts, and factors to be considered when selecting a catalyst were well answered by the majority of candidates.
Parts (d) and (e) about the advantage of heterogeneous catalysts over homogeneous catalysts, and factors to be considered when selecting a catalyst were well answered by the majority of candidates.
Question
Distinguish between a homogeneous and a heterogeneous catalyst.
Other than cost, state one advantage and one disadvantage of using a homogeneous catalyst rather than a heterogeneous catalyst.
Advantage:
Disadvantage:
Other than selectivity and cost, list three factors which should be considered when choosing a catalyst for a particular industrial process.
▶️Answer/Explanation
Markscheme
homogeneous catalyst is in the same phase/state as the reactants and heterogeneous is in a different phase/state to the reactants;
Advantage:
all catalyst exposed to reactants / does not depend on surface area / react more rapidly / OWTTE;
Disadvantage:
difficult to remove from products;
Accept “Cannot always be used at high temperature”.
Apply ECF if C2. (a) the wrong way round.
amount of reactant converted to product per amount of catalyst;
Accept efficiency / conversion rate.
ability to work under different/a range of conditions;
toxicity / environmental/health impact;
catalytic poisoning / active sites become blocked;
lifetime of catalyst;
ease of removal;
Examiners report
This question was relatively well tackled. Almost all knew the difference between the two types of catalysts, though many referred to “state” than the more correct “phase” and could not identify an advantage and a disadvantage and knew of at least some of the factors that need to be considered in decisions relating to the choice of a catalyst.
This question was relatively well tackled. Almost all knew the difference between the two types of catalysts, though many referred to “state” than the more correct “phase” and could not identify an advantage and a disadvantage and knew of at least some of the factors that need to be considered in decisions relating to the choice of a catalyst.
This question was relatively well tackled. Almost all knew the difference between the two types of catalysts, though many referred to “state” than the more correct “phase” and could not identify an advantage and a disadvantage and knew of at least some of the factors that need to be considered in decisions relating to the choice of a catalyst.
Question
Polyacrylonitrile is an important polymer used in the manufacture of carbon fibres. The monomer has the structure below.
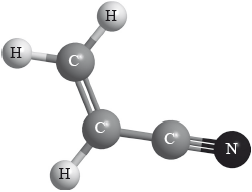
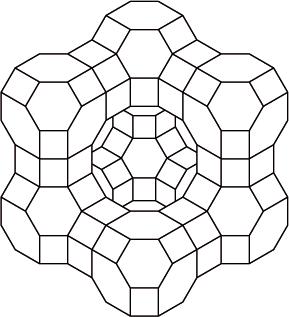
The rate of the polymerization reaction from the gaseous monomer is increased in the presence of a zeolite with the cage structure shown.
A new range of light batteries has been developed that uses open carbon nanotubes, covered with silicon, as electrodes.
Polyacrylonitrile is similar to polypropene and can exist in two forms.
Draw the structure of the isotactic form of polyacrylonitrile showing three repeating units.
Polyacrylonitrile is similar to polypropene and can exist in two forms.
Explain why the isotactic form is more suitable for the manufacture of strong fibres.
Identify the role of the zeolite in the reaction.
Suggest an explanation for its efficiency in favouring the production of the crystalline polymer.
Outline the structure of the open carbon nanotubes.
State a property of these nanotubes that makes them suitable for this use.
▶️Answer/Explanation
Markscheme
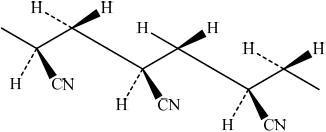 /
/
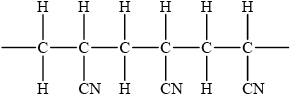 ;
;
Continuation bonds at end of structure needed.
Hydrogen atoms must be included.
Award [1] for chain with CN groups on alternate carbons.
Award [2] for correct chain with CN on alternate carbons with same orientation.
chains pack together better;
strong intermolecular/attractive forces between chains;
chains do not move past each other easily (so fibre strong/rigid);
catalyst;
(selective) because of dimensions/shape/size (of cage);
Accept “large surface area”.
cylinder with hexagons of carbon (atoms);
Accept suitable diagram.
Do not award mark if pentagons are also mentioned.
(electrical) conductor;
Accept low density.
Do not accept light.
Examiners report
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Some very strange polymers were suggested in (a) (i) with the –CN group becoming integrated into the carbon backbone. Candidates were, however, able to explain why the isotactic form is more suitable for fibres. The role of the zeolite in (b) was usually correctly identified but it was not known that the dimensions, size or shape of the cage were an explanation for its efficiency. Most were able to give a good account of carbon nanotubes although some introduced pentagons at the end. This was specifically ruled out in the question – open carbon nano-tubes.
Question
Iron acts as a catalyst in the chemical reactions below.
Reaction I, catalysed by \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\): \({{\text{S}}_{\text{2}}}{\text{O}}_8^{2 – }{\text{(aq)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}} \to {\text{2SO}}_4^{2 – }{\text{(aq)}} + {{\text{I}}_{\text{2}}}{\text{(aq)}}\)
Reaction II, catalysed by Fe(s): \({\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{N}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\)
State the type of catalysis occurring in reaction I.
Outline the mechanism by which each catalyst lowers the activation energy in the reactions above, and state a particular disadvantage of each type of catalysis.
▶️Answer/Explanation
Markscheme
homogeneous;
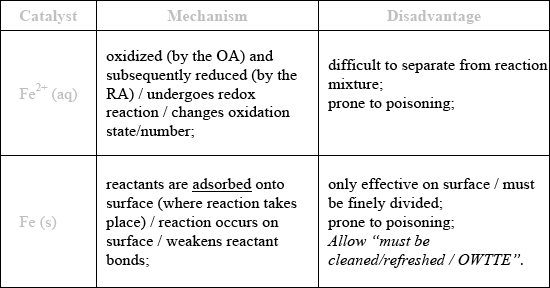
Allow “prone to poisoning” as a disadvantage for either but not both.
Examiners report
Homogeneous catalysis was usually identified in part (a). In (b), few got the correct mechanism for \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\), namely the fact that there is a change in the oxidation state. The command term outline needs to be differentiated from state. Correct disadvantages were usually identified and for Fe(s) most knew that the mechanism involved reactants being adsorbed onto the surface. Outline is an objective 2 command term, as given on P.11 of the guide and equates to giving a brief account or summary. Hence simply stating heterogeneous for the mechanism was incorrect for Fe(s) for example. Candidates need to pay close attention to the command terms as part of their examination preparation.
Homogeneous catalysis was usually identified in part (a). In (b), few got the correct mechanism for \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\), namely the fact that there is a change in the oxidation state. The command term outline needs to be differentiated from state. Correct disadvantages were usually identified and for Fe(s) most knew that the mechanism involved reactants being adsorbed onto the surface. Outline is an objective 2 command term, as given on P.11 of the guide and equates to giving a brief account or summary. Hence simply stating heterogeneous for the mechanism was incorrect for Fe(s) for example. Candidates need to pay close attention to the command terms as part of their examination preparation.
Question
Nanocatalysts have large surface areas per unit mass.
Identify one concern of using nanoscale catalysts.
Explain how zeolites act as selective catalysts.
Carbon nanotubes, which can be produced by the HIPCO process, show great potential as nanocatalysts. Identify the catalyst and conditions used in the HIPCO process.
Catalyst:
Conditions
▶️Answer/Explanation
Markscheme
possible toxicity «of small airborne particles»
OR
unknown health effects
OR
small particle size «and large surface area» may increase reaction rate to dangerous levels
OR
immune system/allergy concerns
OR
uncertain impact on environment
Accept specific health effect (eg. may cause cancer/effect on respiratory system, etc).
pores/cavities/channels/holes/cage-like structures «in zeolites» have specific shape/size
only reactants «with appropriate size/geometry» fit inside/go through/are activated/can react
Catalyst:
iron/Fe
OR
iron«0» «penta» carbonyl/Fe (CO)5
Conditions:
high temperature/any value or range within the range 900–1600 °C
AND
high pressure/any value or range within the range 10–100 atm
Accept “cobalt-molybdenum/Co-Mo/CoMo”.
Accept high pressures expressed in kPa/Pa.
Question
Research has led to the discovery of new catalysts that are in high demand and used in many chemical industries.
Explain, with reference to their structure, the great selectivity of zeolites as catalysts.
Nanocatalysts play an essential role in the manufacture of industrial chemicals.
(i) Describe the high pressure carbon monoxide (HIPCO) method for the production of carbon nanotubes.
(ii) Outline one benefit of using nanocatalysts compared to traditional catalysts in industry.
▶️Answer/Explanation
Markscheme
pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry fit inside/go through/are activated/can react
Accept “molecules/ions” for reactants.
i
iron«0»«penta»carbonyl/Fe(CO)5 catalyst decomposes
OR
Fe(CO)5 (g) → Fe(s) + 5CO(g)
OR
metal nanocatalyst/clusters/particles formed «in situ»
Accept “cobalt-molybdenum/Co-Mo/CoMo” as a catalyst
2CO(g) → CO2(g) +C(s)
Accept “conversion of CO molecules into CNTs/ SWNTs” for M2.
ii
higher efficiency per unit mass/volume of the catalyst «due to higher surface to mass/volume ratio»
OR
greater selectivity «due to metal nanoclusters/surface topology/pore size»
OR
higher stability of the catalyst «due to lower tendency to aggregation»
OR
reduced cost of the catalyst/product/chemicals «as precious metals can be replaced with nanocatalysts made of inexpensive materials»
Accept “high conversion efficiency”.
Accept specific examples such as use of nanocatalysts in fuel cells/catalytic converters «leading to reduced use of Pt/Rh/Pd».
Accept “nanocatalysts often operate under milder conditions «so less energy consumption involved/so promotes principles of green chemistry»”.
Accept “lower energy consumption” OR “reduced carbon footprint” OR “reduced global warming”.
Accept “nanocatalysts often have long lifetimes «so more economical».
Accept “some nanocatalysts have enzyme mimicking activities”.
Question
Antimony oxide is widely used as a homogeneous catalyst in the reaction of benzene-1,4-dicarboxylic acid with ethane-1,2-diol in the production of polyethylene terephthalate (PETE) shown below.
Catalysts reduce the activation energy. Outline how homogeneous catalysts are involved in the reaction mechanism.
Suggest why it is important to know how catalysts function.
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
▶️Answer/Explanation
Markscheme
combine with reactants to form a «temporary» activated complex/intermediate
OR
consumed in one reaction/step AND regenerated in a later reaction/step
[1 mark]
can modify/improve the catalyst/reaction «by making logical predictions»
OR
science relies on models to understand physical reality
Accept other reasonable, relevant answers.
Accept “to predict/select
[1 mark]
electrons AND positive ions «in gaseous state»
high frequency/alternating current passed through argon
OR
«oscillating» electromagnetic/magnetic field
OR
high frequency radio waves
Accept “gas” instead of “argon”.
[2 marks]
Question
Rhodium and palladium are often used together in catalytic converters. Rhodium is a good reduction catalyst whereas palladium is a good oxidation catalyst.
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
Deduce the redox equation for the reaction of nickel(II) chloride solution with the metal identified in (b)(i).
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
▶️Answer/Explanation
Markscheme
carbon monoxide/CO adsorbs onto palladium/Pd
bonds stretched/weakened/broken
OR
«new» bonds formed
OR
activation energy/Ea «barrier» lowered «in both forward and reverse reactions»
products/CO2 desorb «from catalyst surface»
[3 marks]
Fe/iron
OR
Zn/zinc
OR
Co/cobalt
OR
Cd/cadmium
OR
Cr/chromium
Accept “Mn/manganese”.
[1 mark]
Ni2+(aq) + Fe(s) → Ni(s) + Fe2+(aq)
OR
Ni2+(aq) + Zn(s) → Ni(s) + Zn2+(aq)
OR
Ni2+(aq) + Co(s) → Ni(s) + Co2+(aq)
OR
Ni2+(aq) + Cd(s) → Ni(s) + Cd2+(aq)
OR
Ni2+(aq) + Cr(s) → Ni(s) + Cr2+(aq)
Accept “3Ni2+(aq) + 2Cr(s) → 3Ni(s) + 2Cr3+(aq)”.
Do not penalize similar equations involving formation of Fe3+(aq), Mn2+(aq) OR Co3+(aq).
Ignore Cl− ions.
Accept correctly balanced non-ionic equations eg, “NiCl2(aq) + Zn(s) → Ni(s) + ZnCl2(aq)” etc.
Do not allow ECF from (b)(i).
[2 mark]
\(n{\text{(}}{{\text{e}}^ – }{\text{)}}\) «\( = \frac{{2.50{\text{ A}} \times 3600{\text{ s}}}}{{96500{\text{ C}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)» = 0.09326 «mol»
OR
\(n{\text{(Ni)}}\) «\( = \frac{{0.09326{\text{ mol}}}}{2}\)» = 0.04663 «mol»
\(m{\text{(Ni)}}\) «= 0.04663 mol x 58.69 g\(\,\)mol–1» = 2.74 «g»
Award [2] for correct final answer.
[2 marks]
Question
Catalysts can take many forms and are used in many industrial processes.
Suggest two reasons why it might be worth using a more expensive catalyst to increase the rate of a reaction.
▶️Answer/Explanation
Markscheme
Any two of:
greater selectivity
higher efficiency
longer life expectancy
OR
not easily poisoned
easier to recover
low«er» environmental impact
large range of conditions/temperatures/pressures supported
lower energy costs
increase in yield «per unit time» offsets cost of catalyst
[Max 2 Marks]
Examiners report
Question
Both HDPE (high density polyethene) and LDPE (low density polyethene) are produced by the polymerization of ethene.
Both of these are thermoplastic polymers. Outline what this term means.
Compare and contrast the structures of HDPE and LDPE.
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
▶️Answer/Explanation
Markscheme
soften/melt when heated
OR
can be melted and moulded
Accept “low melting point” OR “can be moulded when heated”.
[1 mark]
both have «long» hydrocarbon chains
OR
both have chains comprising CH2 units
HDPE has little/no branching AND LDPE has «more» branching
Accept “CH2–CH2 units”.
Accept “HDPE more crystalline”.
[2 marks]
HDPE is more rigid/less flexible
OR
HDPE has a higher melting point
OR
HDPE has greater «tensile» strength
Accept “HDPE has lower ductility”.
[1 mark]
form «temporary» activated complexes/reaction intermediates
Accept “consumed in one reaction/step AND regenerated in a later reaction/step”.
Accept “provides alternative mechanism”.
[1 mark]
inductively coupled plasma/ICP spectroscopy using mass spectroscopy/mass spectrometry/MS/ICP-MS
OR
inductively coupled plasma/ICP spectroscopy using optical emission spectroscopy/OES/ICP-OES
Accept “atomic absorption/aa spectroscopy” or “MS/massspectroscopy/mass spectrometry”.
[1 mark]
Any two of:
many types «of plastics» exist
OR
«plastics» require sorting «by type»
«plastics» need to be separated from non-plastic materials
OR
«often» composites/moulded on/bound to non-plastic/other components
Accept other valid factors such as thermal decomposition of some plastics, production of toxic fumes, etc.
[2 marks]
«different classifications are appropriate for» different properties/applications/purposes
[1 mark]
Question
Inductively Coupled Plasma (ICP) used with Mass Spectrometry (MS) or Optical Emission Spectrometry (OES) can be used to identify and quantify elements in a sample.
The following graphs represent data collected by ICP-OES on trace amounts of vanadium in oil.
Graph 1: Calibration graph and signal for 10 μg kg−1 of vanadium in oil
Graph 2: Calibration of vanadium in μg kg−1
[Source: © Agilent Technologies, Inc.1998. Reproduced with Permission, Courtesy of Agilent Technologies, Inc.]
ICP-OES/MS can be used to analyse alloys and composites. Distinguish between alloys and composites.
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.
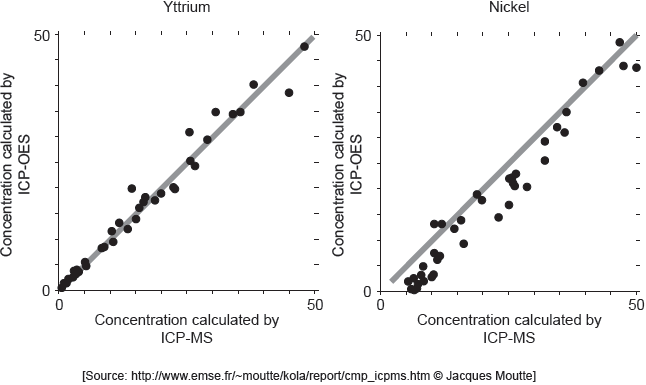
Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
Identify the purpose of each graph.
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
Vanadium(V) oxide is used as the catalyst in the conversion of sulfur dioxide to sulfur trioxide.
SO2(g) + V2O5(s) → SO3(g) + 2VO2(s)
\(\frac{1}{2}\)O2(g) + 2VO2(s) → V2O5(s)
Outline how vanadium(V) oxide acts as a catalyst.
▶️Answer/Explanation
Markscheme
Alloy:
mixture of metal with other metals/non-metals
OR
mixture of elements that retains the properties of a metal
Composite:
reinforcing phase embedded in matrix phase
Award [1 max] for implying “composites only have heterogeneous/nonhomogeneous compositions”.
[2 marks]
effective for yttrium «but less/not for nickel»
points on nickel graph do not lie on «y = x» line
OR
cannot be used for low concentrations of nickel
OR
concentration of nickel is lower than recorded value
Accept “ICP-OES is more accurate for lower yttrium concentrations than higher concentrations” for M1.
Accept [Ni] and [Y] for concentrations of nickel and yttrium.
Accept “detection limit for yttrium is lower than for nickel” for M2.
Award [1 max] for “more accurate for yttrium at lower concentrations AND nickel at higher concentrations”.
[2 marks]
Graph 1: determines wavelength of maximum absorption/maximum intensity «for vanadium»
Graph 2: determines absorption of known concentrations «at that wavelength»
OR
estimates [V]/concentration in a sample using «the signal» intensity
Do not accept just “determines maximum wavelength/λmax” for M1.
Do not accept “calibration curve” for M2.
[2 marks]
«14 950 = 392.19x + 147.62»
x = 37.74 «μg kg–1»
Answer must be given to four significant figures.
Do not accept values obtained directly from the graph.
[1 mark]
vanadium reduced in first reaction AND oxidized in second reaction
OR
V2O5 oxidizes SO2 in first reaction AND VO2 reduces O2 in second reaction
OR
vanadium returns to original oxidation state «after reaction»
provides an alternative reaction pathway/mechanism «with a lower activation energy» ✔
Do not accept “reactants adsorb onto surface AND products desorb”.
Accept “oxidation number” for “oxidation state”.
[2 marks]

