Question
New materials have brought many benefits to society but come with associated risks.
(a) High-pressure carbon monoxide disproportionation (HiPco) produces carbon atoms that react with nano catalysts to produce carbon nanotubes.
(i) Write the equation for the disproportionation of carbon monoxide to produce carbon atoms.
(ii) Calculate the percent atom economy of producing carbon using this method. Use section 1 of the data booklet.
(iii) Outline how a metal functions as a heterogeneous catalyst.
(iv) Explain whether the production of carbon nanotubes using HiPco is a bottom up or top down nanotechnology technique.
(v) Suggest one health risk of using nanoparticles.
(b) Kevlar® is a recyclable polyamide polymer and a liquid crystal. One repeating unit of the polyamide is shown.
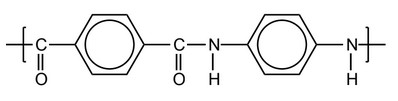
(i) Outline what is meant by a liquid crystal.
(ii) Some liquid crystal displays (LCD) use liquid crystals between two polarizing filters. The display appears black until a small voltage is applied. Outline how the liquid crystals allow polarized light to pass through the filters.
(iii) Identify the resin identification code (RIC) that applies to Kevlar®. Use section 30 of the data booklet.
The IR spectrum of Kevlar® is shown.
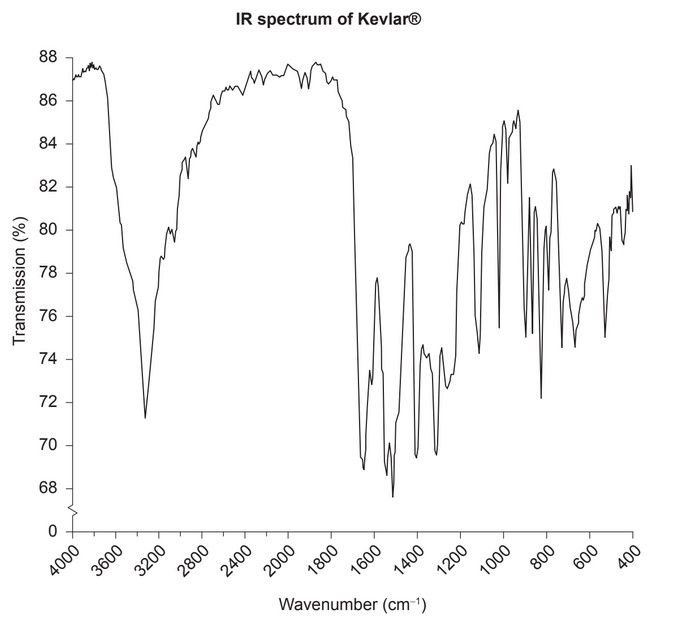
(iv) Deduce the peak in the Kevlar® IR spectrum which would not be found in compounds with any other RIC code. Use Sections 26 and 30 of the data booklet.
(v) Kevlar® is a condensation polymer. Distinguish between addition and condensation polymerization, in terms of monomers and products.
Monomers:
Products:
Answer/Explanation
Answer:
(a) \(2CO(g) \rightarrow C(s) + CO_2(g)\)
(ii) «100 × 12.01 / 2 × (12.01+16.00) = » 21.44%
(iii) «gaseous» reactants adsorb onto «metal» surface
OR
catalyst provides surface for reaction to occur
weakens «reactant» bonds
OR
products desorb
(iv) bottom up AND molecular assembly «rather than decomposition»
(v) Any one of:
more easily airborne/inhaled
have similar dimensions as biological molecules/interfere with biochemical
reactions
easily absorbed into body
may cross cell membranes
large surface area could increase toxicity
human defence system not effective with small size
(b) (i) fluids with «some» properties that are anisotropic/depend on molecular orientation «relative to a fixed axis»
(ii) polar «molecules»
change orientation upon application of electric field
OR
«in some orientations» molecules rotate plane of polarization «of polarized light»
(iii) 7
(iv) 3300 to 3500
(v) Monomers:
addition: unsaturated/containing C=C/C≡C
condensation: monomers have two reactive sites/functional groups
Products:
addition: one product/no by-products AND
condensation: small molecule/HCl eliminated/two products
Question
There has been significant growth in the use of carbon nanotubes, CNT.
a. Explain these properties of carbon nanotubes.
b(i)CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more useful than Type 1.
b(iiExplain the role of electrons in superconducting materials in terms of the Bardeen-Cooper-Schrieffer (BCS) theory.
c(i)Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium-CNT alloy.
c(ii)P[ure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
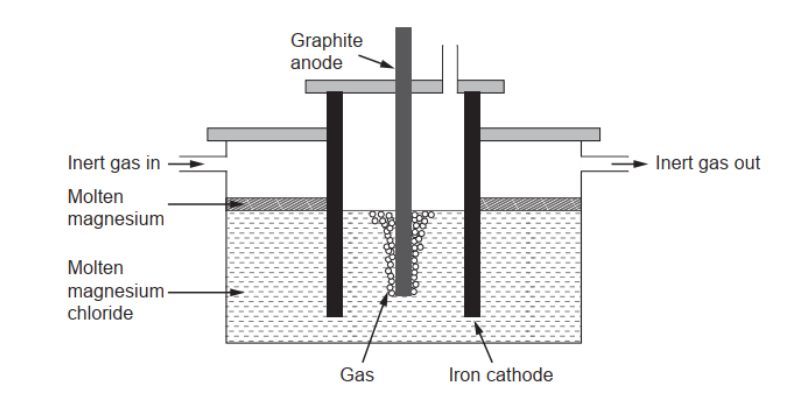
Calculate the theoretical mass of magnesium obtained if a current of $3.00 \mathrm{~A}$ is used for 10.0 hours. Use charge $:(Q)=$ current $(I) \times$ time $(t)$ and section 2 of the data booklet.
c(iii)Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
d. Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
e. Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
▶️Answer/Explanation
Markscheme
a. Excellent strength: defect-free $A N D$ rigid/regular $2 \mathrm{D} / 3 \mathrm{D}$
Excellent conductivity: delocalized electrons
Accept “carbons/atoms are all covalently bonded to each other” for M1.
$\mathrm{b}(\mathrm{i})$ Any two of:
have higher critical temperatures/ $T_{\mathrm{c}}$ «than Type 1 »
OR
can act at higher temperatures
have higher critical magnetic fields $/ B_{\mathrm{c}}$ «than Type $1 »$
less time needed to cool to operating temperature
less energy required to cool down/maintain low temperature
$\mathrm{b}(\mathrm{ii})$ Any three of:
passing electrons «slightly» deform lattice/displace positive ions/cations
electrons couple/form Cooper pairs/condense with other electrons
energy propagates along the lattice in wave-like manner/as phonons
Cooper pair/electron condensate/pair of electrons moves through lattice freely OR
phonons are «perfectly» elastic/cause no energy loss
c(i)Any of:
ductility
strength/resistance to deformation $\boldsymbol{V}$
malleability
hardness
resistance to corrosion/chemical resistance
range of working temperatures
density $\sim$
Do not accept “conductivity”.
$$
\begin{aligned}
& \text { c(ii) } s Q=I \times t=3.00 \times 10.0 \times 3600=» 108000 \mathrm{C} \checkmark \\
& « \frac{Q}{F}=\frac{108000 \mathrm{C}}{96500 \mathrm{Cmol}^{-1}}=» 1.12 \ll \mathrm{mol} \mathrm{e}^{-} » \\
& \text { « } \frac{1.12 \mathrm{~mol}}{2}=0.560 \mathrm{~mol} \mathrm{Mg} » \\
& \left\langle m=0.560 \mathrm{~mol} \times 24.31 \mathrm{~g} \mathrm{~mol}^{-1}=» 13.6 \ll \mathrm{g}\right\rangle \\
&
\end{aligned}
$$
Award [3] for correct final answer.
c(iii)Argon/Ar/helium/He
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen $/ \mathrm{N}_2$ “.
d. pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react
Accept “molecules/ions” for “reactants” in M2.
e. rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order AND have «some» directional order/pattern
Accept “linear” for “rod-shaped”.
