Question
Proteins are large polymers of 2-amino acids.
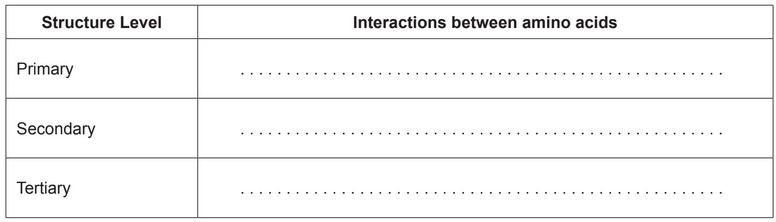
(a) Describe the interactions between amino acids occurring at the primary, secondary and tertiary levels within a protein.
(b) Explain how paper chromatography can separate and identify mixtures of amino acids.
(c) Explain the concept of product inhibition in metabolic pathways.
The following diagram shows a protein calibration curve.
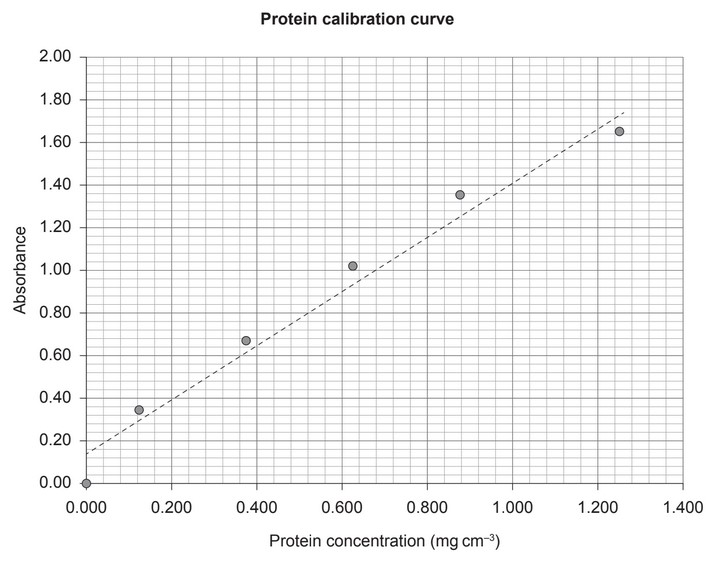
(d) State the concentration, in mg \(cm^{-3}\), of a protein sample with an absorbance of 0.80.
Answer/Explanation
Answer:
(a) 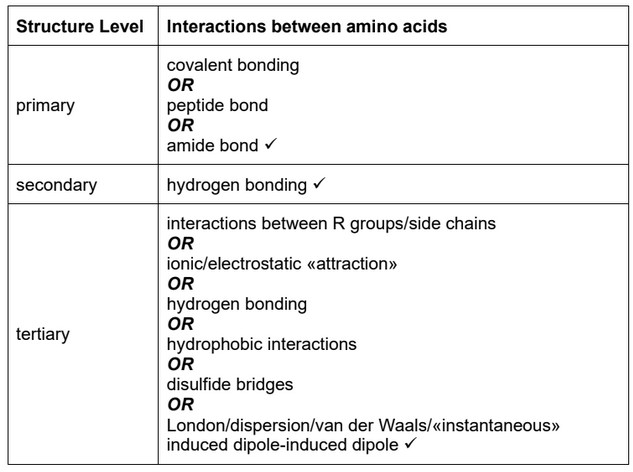
(b) Any two of:
sample spotted on paper/stationary phase AND solvent moves up the paper
OR
continuous cycles of adsorption and desorption/dissolution
OR
analyte moves when in solvent AND does not move when on paper
different/depends on attractions to mobile phase AND stationary phase/paper
OR
«amino acids» separated based on solubilities in/affinity to the two phases
OR
separated based on polarities/polar attractions/molar masses of «amino acids»
developed with ninhydrin/reagent/locating agent
OR
become identified with UV «light»
«amino acids» identified by Rf/retention factor «values»
OR
Rf/retention factors «values» compared with known samples
(c) product of reaction is inhibitor of enzyme
OR
product binds/bonds to allosteric site of enzyme
regulates own production
OR
sets up feedback loop to control concentration/production
(d) 0.520 «mg \(cm^{-3}\)»
Question
a(i)Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
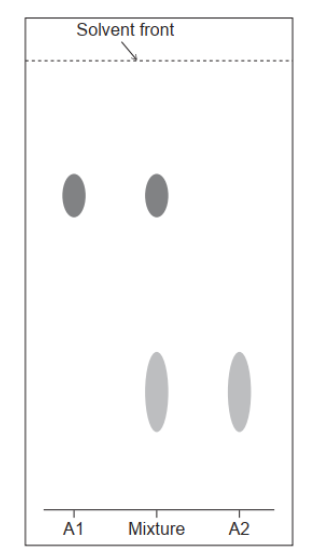
Determine the $R_f$ value of $A 1$.
a(ii).roteins are polymers of amino acids.
The mixture is composed of glycine, Gly, and isoleucine, Ile. Their structures can be found in section 33 of the data booklet. Deduce, referring to relative affinities and $R_f$, the identity of $A 1$.
b. Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the $\alpha$-helix in the secondary structure.
c(i)Proteins are polymers of amino acids.
Sketch and label two oxygen dissociation curves, one for adult hemoglobin and one for foetal hemoglobin.
c(ii).roteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
▶️Answer/Explanation
Markscheme
$\mathrm{a}(\mathrm{i}) 0.70$
Accept any value within the range “0.67-0.73”.
a(ii)le $\boldsymbol{A N D}$ larger $\mathrm{R}_{\mathrm{f}}$
more soluble in non-polar solvent «mobile phase»
OR
not as attracted to polar «stationary» phase
Only award M2 if lle is identified in M1.
b. hydrogen/H bonding «between amido hydrogen and carboxyl oxygen atoms»
c(i).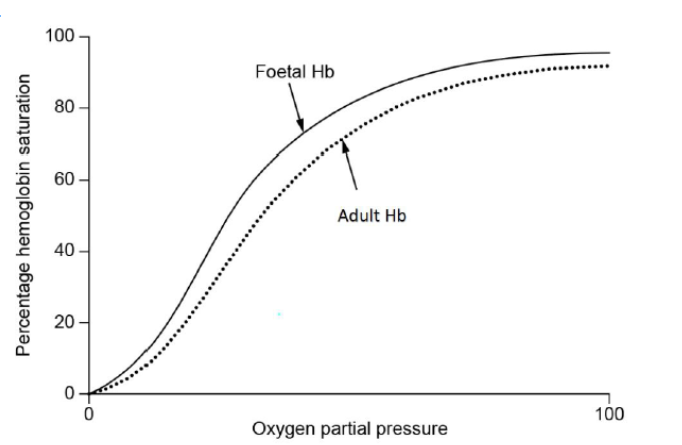
both curves sigmoidal shape AND starting at zero
foetal hemoglobin showing greater affinity/steeper/higher gradient
Do not penalize if convergence is not approached for $M 1$.
Both curves must be labelled to score M2.
c(ii)Any two of:
contains two gamma/ $\gamma$ units «instead of two beta/ $\beta$ units found in adults» OR
differs in amino acid sequence «from the two beta// $\beta$ units found in adults» less sensitive to inhibitors/2,3-BPG/DPG
receives $\mathrm{O}_2$ from «partly deoxygenated» blood so can work at low $\mathrm{pO}_2$
low $\mathrm{p} \mathrm{CO}_2$ in foetal blood increases affinity for $\mathrm{O}_2$
hemoglobin concentration in foetal blood greater than in the mother
