Question
\text { The main fatty acid composition of cocoa butter and coconut oil is detailed below. }
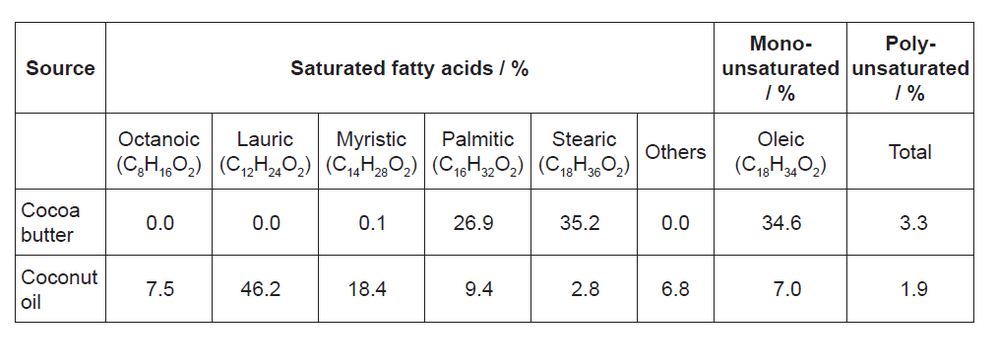
a. The melting points of cocoa butter and coconut oil are $34^{\circ} \mathrm{C}$ and $25^{\circ} \mathrm{C}$ respectively.
Explain this in terms of their saturated fatty acid composition.
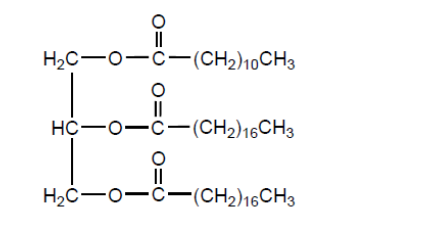
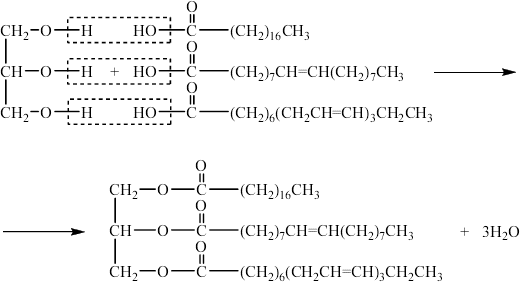
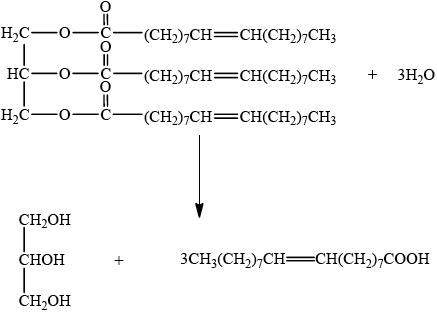
b. Fats contain triglycerides that are esters of glycerol and fatty acids. Deduce an equation for the acid hydrolysis of the following triglyceride.
c. The addition of partially hydrogenated cocoa butter to chocolate increases its melting point and the content of trans-fatty acids (trans-fats).
Outline two effects of trans-fatty acids on health.
▶️Answer/Explanation
Markscheme
a. coconut oil has higher content of lauric/short-chain «saturated» fatty acids
OR
cocoa butter has higher content of stearic/palmitic/longer chain «saturated» fatty acids $[\boldsymbol{W}]$
longer chain fatty acids have greater surface area/larger electron cloud $[\boldsymbol{\sim}]$
stronger London/dispersion/instantaneous dipole-induced dipole forces «between triglycerides of longer chain saturated fatty acids» [ $\boldsymbol{\sim}$ ]
Note: Do not accept arguments that relate to the melting points of saturated and unsaturated fats.
b.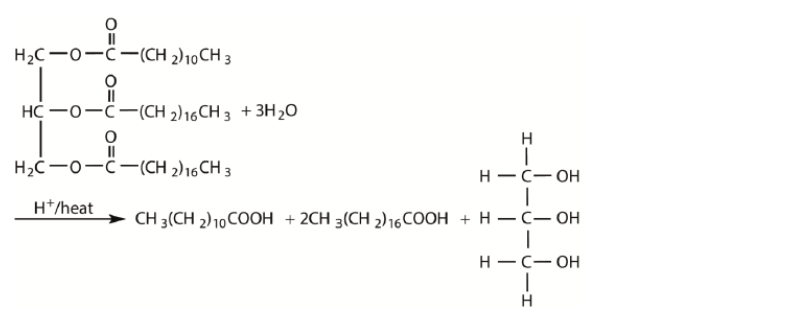
correct products $[\boldsymbol{V}$ ]
correctly balanced $[\boldsymbol{V}]$
c. Any two of:
«increased risk of» coronary/heart disease $[\checkmark]$
«increased risk of» stroke $[\boldsymbol{\sim}]$
«increased risk of» atherosclerosis $[\boldsymbol{\sim}]$
«increased risk of type-2» diabetes $[\boldsymbol{V}]$
increase in LDL cholesterol $[\boldsymbol{V}]$
decrease in HDL cholesterol $[\boldsymbol{\sim}]$
«increased risk of» obesity $[\boldsymbol{\sim}]$
Question
Phosphatidylcholine may be formed from propane-1,2,3-triol, two lauric acid molecules, phosphoric acid and the choline cation.
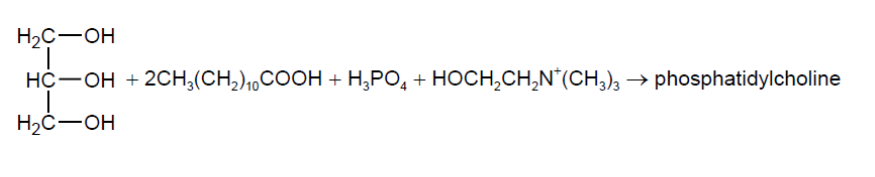
a(i)Deduce the structural formula of phosphatidylcholine.
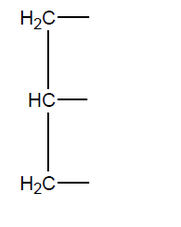
a(ii)dentify the type of reaction in (a).
b. Lecithin is a major component of cell membranes. Describe the structure of a cell membrane.
c. Predict, giving a reason, the relative energy density of a carbohydrate and a lipid of similar molar mass.
d. Lecithin aids the body’s absorption of vitamin $E$.
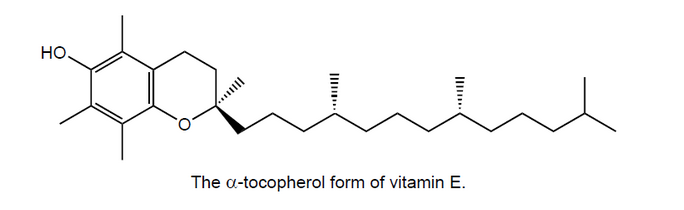
Suggest why vitamin $E$ is fat-soluble.
e. Phospholipids are also found in lipoprotein structures.
Describe two effects of increased levels of low-density lipoprotein (LDL) on health.
▶️Answer/Explanation
Markscheme
a(i).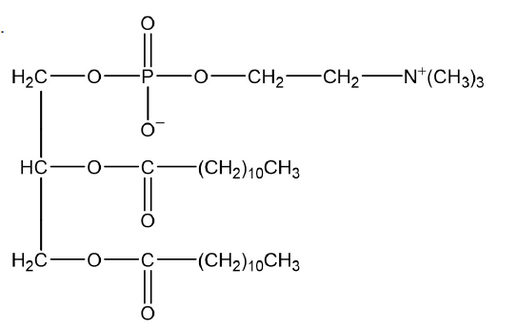
phosphodiester correctly drawn [ $\checkmark$ ]
both ester groups correctly drawn [ $\boldsymbol{V}]$
Note: Accept protonated phosphate.
Accept phosphodiester in centre position.
a(iicondensation [ $\checkmark$ ]
Note: Accept “esterification”.
Accept “nucleophilic substitution/ $\mathrm{S}_{\mathrm{N}}$ “.
b. phospholipid bilayer/double layer
OR
two layers of phospholipids [ $\boldsymbol{V}$ ]
polar/hydrophilic heads facing aqueous environment $A N D$ non-polar/hydrophobic tails facing away from aqueous environment [ $\boldsymbol{C}$ ]
Note: Award [2] for a suitably labelled diagram.
Award [1 max] for a correct but unlabelled diagram.
Accept “polar/hydrophilic heads on outside AND non-polar/hydrophobic tails on inside for M2.
c. carbohydrates less energy dense $A N D$ carbohydrates higher ratio of oxygen to carbon/more oxidized/less reduced [ $\sim$ ]
d. long non-polar/hydrocarbon chain «and only one hydroxyl group»
OR
forms London/dispersion/van der Waals/vdW interactions with fat [ $\boldsymbol{U}$ ]
Note: Accept “alcohol/hydroxy/OH” for “hydroxyl” but not “hydroxide”.
e. atherosclerosis/cholesterol deposition «in artery walls» [ $\boldsymbol{\sim}]$
increases risk of heart attack/stroke/cardiovascular disease/CHD [ $]$
Note: Accept “arteries become blocked/walls become thicker”, “increases blood pressure”, or “blood clots”.
Do not accept “high cholesterol”.
Question
Stearic acid $\left(M_{\mathrm{r}}=284.47\right)$ and oleic acid $\left(M_{\mathrm{r}}=282.46\right)$ have the same number of carbon atoms. The structures of both lipids are shown in section 34 of the data booklet.
a. The iodine number is the number of grams of iodine which reacts with $100 \mathrm{~g}$ of fat. Calculate the iodine number of oleic acid.
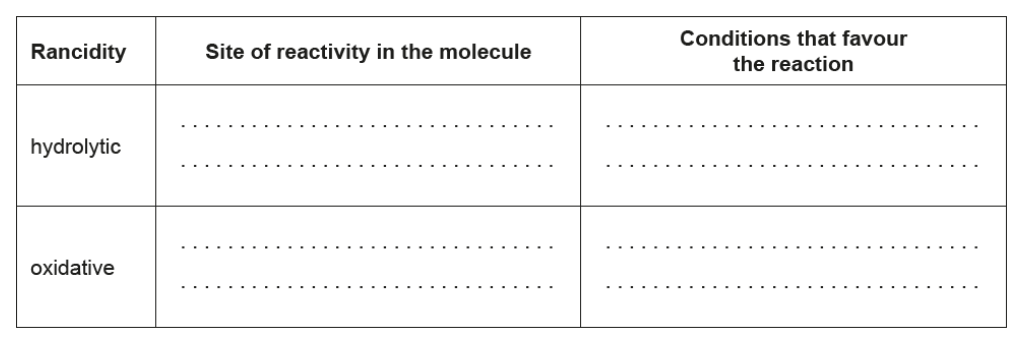
b. The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste. Compare hydrolytic and oxidative rancidity.
c. State one similarity and one difference in composition between phospholipids and triglycerides.
Similarity:
Difference:
▶️Answer/Explanation
Markscheme
a. «one $\mathrm{C}=\mathrm{C}$ bond»
«1 mole iodine : 1 mole oleic acid»
” $\frac{100 \times 253.80}{282.46}=89.85$ «g of $\mathrm{I}_2$ “
NOTE: Accept “90 “g of $\mathrm{I}_2$ »”.
b.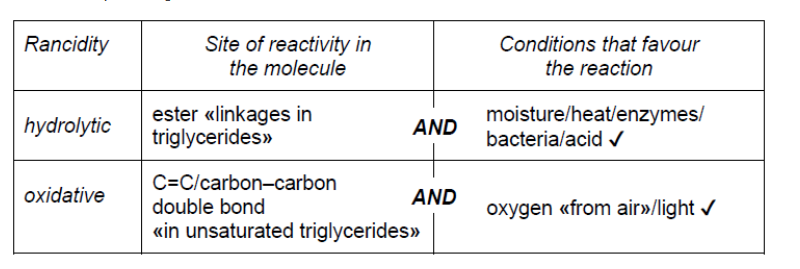
NOTE: Award [1] for any two sites or conditions from any of the four listed.
Accept “high temperature” for “heat”. Accept “lipase” for “enzyme”.
Do not accept just “double bond”.
Accept “air” for “oxygen” and “UV/sun” for “light”.
Ignore any reference to heat/high temperature as a condition for oxidative.
c. Similarity:
«derived from» propane-1,2,3-triol/glycerol/glycerin/glycerine
OR
«derived from» at least two fatty acids
OR
contains ester linkages
OR
long carbon chains
NOTE: Do not accept “two fatty acids as both a similarity and a difference”.
Do not accept just “hydrocarbon/carbon chains”.
Difference:
phospholipids contain two fatty acids «condensed onto glycerol» $A N D$ triglycerides three
OR
phospholipids contain phosphate/phosphato «group»/residue of phosphoric acid $A N D$ triglycerides do not
NOTE: Accept “phospholipids contain phosphorus AND triglycerides do not”.
Accept “phospholipids are amphiphilic AND triglycerides are not” OR “phospholipids have hydrophobic tails and hydrophilic heads AND triglycerides do not”.
Question
Lipids play several roles in our bodies.
a. A phospholipid generally consists of two hydrophobic fatty acids and a hydrophilic group.
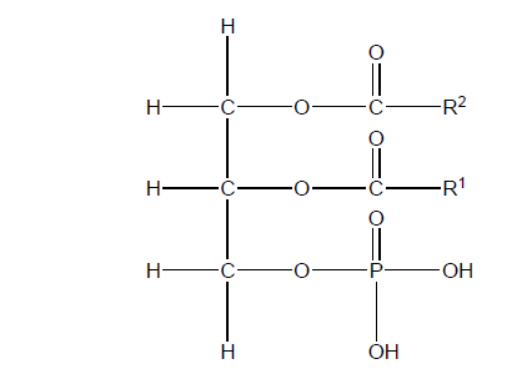
Fatty acids are products of the acidic hydrolysis of phospholipids. Deduce the names of the other two products.
b.i. The iodine number is the maximum mass of iodine that reacts with $100 \mathrm{~g}$ of an unsaturated compound.
Determine the iodine number of stearidonic acid, $\mathrm{C}_{17} \mathrm{H}_{27} \mathrm{COOH}$.
b.ii.State two functions of lipids in the body.
c. Outline one effect of increased levels of low-density lipoproteins in the blood.
▶️Answer/Explanation
Markscheme
a. phosphoric acid
glycerol/propane-1,2,3-triol
Do not accept formulas.
b.i. ALTERNATIVE 1:
$4 \mathrm{C}=\mathrm{C}$ bonds/ 4 carbon to carbon double bonds
mass of iodine per mole of acid $=\ll 4 \times 253.80 \mathrm{~g} \mathrm{~mol}^{-1}=» 1015.2$ « mol${ }^{-1} »$
iodine number $«=\frac{1015.2 \mathrm{~g} \mathrm{~mol}^{-1}}{276.46 \mathrm{~g} \mathrm{~mol}^{-1}} \times 100 »=367$
ALTERNATIVE 2:
$4 \mathrm{C}=\mathrm{C}$ bonds $/ 4$ carbon to carbon double bonds
” $\frac{100 \mathrm{~g}}{276.46 \mathrm{~g} \mathrm{~mol}^{-1}} \times 4=$ = $1.447 \mathrm{~mol}$ of $\mathrm{I}_2$ «reacts with $100 \mathrm{~g}$ »
iodine number $«=1.447 \mathrm{~mol} \times 253.80 \mathrm{~g} \mathrm{~mol}^{-1} »=367 \checkmark$
Award [3] for correct final answer.
b.ii Any two of:
«structural» components of cell membranes
energy storage/utilization
«thermal/electrical» insulation
transport «of lipid-soluble molecules»
hormones/chemical messengers
Accept other specific functions, such as “prostaglandin/cytokine/bile acid synthesis”, “cell differentiation/growth”, “myelination”, “storage of vitamins/biomolecules”, “signal transmission”, “protection/padding of organs”, “precursors/starting materials for the biosynthesis of other lipid”.
c. Any one of:
atherosclerosis/cholesterol deposition «in artery walls»
heart/cardiovascular disease
stroke
Accept “arteries become blocked/walls become thicker”.
Question
Lipids provide energy and are an important part of a balanced diet.
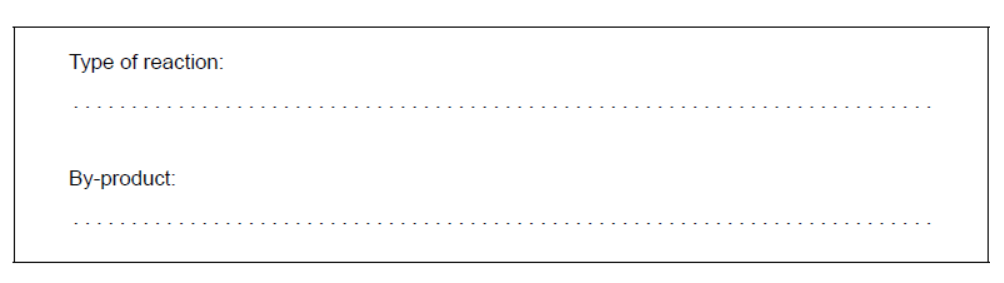
a. Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
b. Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon-carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid $M_{\mathrm{r}}=304.5$ )
c. Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
d. Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
e. Determine, to the correct number of significant figures, the energy produced by the respiration of $29.9 \mathrm{~g}$ of $\mathrm{C}_5 \mathrm{H}_{10} \mathrm{O}_5$.
$$
\Delta H_c\left(\mathrm{C}_5 \mathrm{H}_{10} \mathrm{O}_5\right)=205.9 \mathrm{~kJ} \mathrm{~mol}^{-1}
$$
f. Explain why lipids provide more energy than carbohydrates and proteins.
▶️Answer/Explanation
Markscheme
a. Type of reaction:
condensation
OR
esterification/triesterification
OR
nucleophilic substitution/nucleophilic displacement/ $\mathrm{S}_{\mathrm{N}} 2$
By-product:
water $/ \mathrm{H}_2 \mathrm{O}$
Do not accept just “substitution/displacement”.
[2 marks]
b. ALTERNATIVE 1
$$
\begin{aligned}
& \text { « } \frac{334}{253.8}=» 1.32 \text { AND « } \frac{100}{304.5}=\geqslant 0.328 \\
& \qquad \frac{1.32}{0.328} \approx \$ 4
\end{aligned}
$$
ALTERNATIVE 2
$$
\begin{aligned}
& « 334 \times \frac{304.5}{100} \approx 11017 \\
& « \frac{1017}{253.8} \approx \rrbracket 4
\end{aligned}
$$
Award [2] for correct final answer.
[2 marks]
c.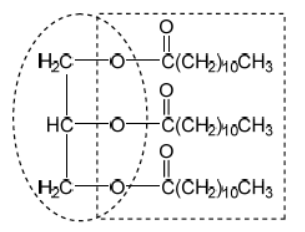
glycerol backbone
ester formula AND linkage
Accept a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Accept condensed formula for ester.
[2 marks]
d. has affected consumption of trans-fats/cis-fats/saturated fats/unsaturated fats/hydrogenated/artificially altered fats
OR
reduce/eliminate trans-fats/increase in cis-fats
OR
reduce/eliminate saturated fats
OR
increase unsaturated fats
Do not accept “decrease in fat” alone.
Accept “lipid” for “fats”.
[1 mark]
e. $« \frac{29.9 \mathrm{~g}}{150.15 \mathrm{~g} \mathrm{~mol}^{-1}}=» 0.199$ «mol»
$« 0.199 \mathrm{~mol} \times 205.9 \mathrm{~kJ} \mathrm{~mol}^{-1}=» 41.0$ «kJ»
Ignore significant figures in $M 1$.
Award [2] for correct final answer.
Award [1 max] for incorrect significant figures in final answer.
[2 marks]
f. ratio of oxygen to carbon in lipids lower
OR
lipids less oxidized
OR
lipids more reduced
more energy per mass/g released when lipids are oxidized
Accept “raverage” oxidation number of carbon in linoleic acid is lower” for M1.
[2 marks]
Question
Many lipids are found in the human body. One type of lipid is a triglyceride.
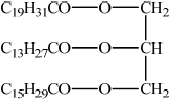
Steroids and phospholipids are both classes of lipid found in the body. Cholesterol is a steroid. A structure of lecithin, a phospholipid, is shown below.
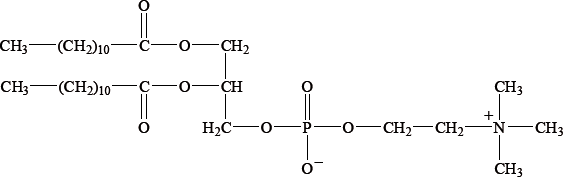
The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the equation for the reaction between glycerol and stearic acid to form a triglyceride.
Compare the structures of the two fatty acids: linoleic and linolenic acids.
State why these two fatty acids are so important in the human diet.
Distinguish between HDL and LDL cholesterol.
Compare the composition of cholesterol with a phospholipid such as lecithin.
Determine whether cholesterol or lecithin is more soluble in water.
▶️Answer/Explanation
Markscheme
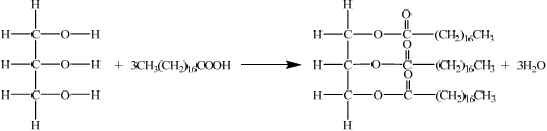
correct structure of glycerol and correct formula of stearic acid;
correct structure of triglyceride;
\({\text{3}}{{\text{H}}_{\text{2}}}{\text{O}}\) and coefficient of 3 on stearic acid;
Accept displayed or condensed formulas for molecules.
both have first double bond on C9 with carbon / linoleic has an \(\omega – 6{\text{ C=C}}\) double bond and linolenic acid has an \(\omega – 3{\text{ C=C}}\) double bond;
linoleic acid has 2 double carbon bonds and linolenic acid 3 double carbon bonds;
fatty acids are essential / body cannot synthesize them / OWTTE;
LDL is (a) larger (molecule) than HDL;
LDL transports cholesterol to arteries and HDL removes cholesterol from arteries;
LDL produced from saturated fats/trans fatty acids;
LDL increases the risk of heart disease/problems;
Accept converse statements for HDL.
Do not accept LDL is bad cholesterol and HDL is good cholesterol.
cholesterol is composed of C, H and O only and phospholipid contains C, H, O, P and N;
lecithin;
Examiners report
Candidates could not write an equation for the reaction between glycerol and stearic acid to form a triglyceride. Where candidates did write the correct equation they often did not balance the equation correctly.
In part (b) many candidates did not correctly recognize the difference in the number of carbon – carbon double bonds in the two fatty acids, nor the location of the double bonds and hence the significance of the omega-3 and omega-6 terminology.
Some candidates correctly identified that these fatty acids cannot be synthesised by the body and hence are essential.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
Question
Cholesterol belongs to a class of substances named lipids.
Identify the characteristic structural feature of cholesterol.
Identify two other types of lipids found in the human body.
State what the terms HDL and LDL represent.
Compare the structures of linoleic acid and linolenic acid.
▶️Answer/Explanation
Markscheme
steroid/steroidal backbone/4 ring/tetra cyclic carbon structure skeleton;
Do not accept OH, hydroxyl, hydroxide, alcohol.
Accept a correct sketch of the steroid backbone.
phospholipids;
triglycerides/triglycerols;
high density lipoprotein and low density lipoprotein;
both have 18 carbon atoms;
both have carboxyl/COOH;
linoleic acid has 2 double bonds and linolenic 3 / linoleic acid has less double bonds / linoleic acid is less unsaturated;
first double bond of linoleic is after the \({{\text{6}}^{{\text{th}}}}\) C atom and first of linolenic is after the \({{\text{3}}^{{\text{rd}}}}\) C atom from the end of the \({\text{C}}{{\text{H}}_{\text{3}}}\) group/counting from side of the chain that does not have COOH group / linoleic acid is omega-6 and linolenic acid is omega-3 / OWTTE;
Examiners report
Most candidates identified the steroid backbone.
Many candidates named only one other type of lipid.
Some candidates stated correctly the terms of HDL and LDL.
Most candidates compared at least two features of the structures of linoleic and linolenic acid.
Question
Describe the chemical composition of a triglyceride.
The following two structures represent isomers of a fatty acid.
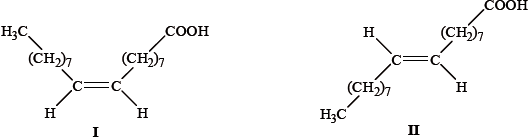
State and explain which isomer has the higher melting point.
▶️Answer/Explanation
Markscheme
ester of glycerol/propan-1,2,3-triol and three fatty acids/long chain carboxylic acids;
II;
more straight molecule/greater surface area hence greater distortion of electron cloud / allows closer packing of fatty acids for trans / does not allow closer packing for cis isomers / OWTTE;
trans greater van der Waals’ forces / cis less van der Waals’ forces;
Accept London/dispersion forces.
Examiners report
Many candidates knew the structure of triglycerides.
Most candidates identified structure II as the one with the highest melting point and many gave the correct reason.
Question
Malnutrition can be caused by starvation, dieting or a person eating an excess of highly processed food.
Describe the structural composition of the following nutrients:
fats and oils
monosaccharides.
Liver is a source of arachidonic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{{\text{(CH=CHC}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{2}}}{\text{COOH}}\), and fish oils are a source of linolenic acid. With reference to the structure of linolenic acid in Table 22 of the Data Booklet, explain why arachidonic acid has a much lower melting point compared to linolenic acid, even though it contains two more carbon atoms.
▶️Answer/Explanation
Markscheme
(tri)esters/contains COO group/(tri)glycerides;
(three) fatty acid chains joined to glycerol/propan-123-triol / OWTTE;
Accept long-chain carboxylic acid and glycerine.
empirical formula is \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\) / general formula is \({{\text{C}}_n}{{\text{H}}_{{\text{2}}n}}{{\text{O}}_n}\);
contains one carbonyl/C=O group and at least two/several hydroxyl/OH groups;
four C=C bonds in arachidonic acid and three C=C bonds in linolenic acid / greater unsaturation/number of C=C bonds in arachidonic acid;
presence of double bonds prevents close-packing/kinks in structure / extra double bond decreases ability of arachidonic acid molecules to align themselves together / OWTTE;
(so) van der Waals’/London/dispersion/intermolecular forces weaker in arachidonic acid;
Examiners report
The descriptions of structure in (b) often lacked the essential points – perhaps some candidates did not know how to interpret “structural composition”.
the descriptions of structure in (b) often lacked the essential points – perhaps some candidates did not know how to interpret “structural composition”.
The effect of double bonds on the intermolecular forces in unsaturated fats was well known in (c), and thankfully few candidates referred to the breaking of covalent bonds.
Question
Unsaturated fats contain C=C double bonds. The amount of unsaturation in a fat or oil can be determined by titrating with iodine solution.
(a) Define the term iodine number.
(b) Linoleic acid (\({M_{\text{r}}} = 281\)) has the following formula.
\({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{\text{CH=CHC}}{{\text{H}}_{\text{2}}}{\text{CH=CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{7}}}{\text{COOH}}\)
Calculate the volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) iodine solution required to react exactly with 1.00 g of linoleic acid.
▶️Answer/Explanation
Markscheme
(a) the number of grams/mass of iodine that add to/react with 100 g of the fat/lipid/oil;
(b) amount of linoleic acid \( = \frac{1}{{281}} = 0.00356{\text{ (mol)}}\);
amount of \({{\text{I}}_{\text{2}}}\) required \( = 2 \times 0.00356 = 0.00712{\text{ (mol)}}\);
volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) solution \( = 7.12{\text{ c}}{{\text{m}}^{\text{3}}}{\text{/0.00712 d}}{{\text{m}}^{\text{3}}}\);
OR
281 g of acid require 507.6 g of iodine;
1 g of acid requires 1.806 g/0.00712 mol of iodine;
volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) solution \( = 7.12{\text{ c}}{{\text{m}}^{\text{3}}}{\text{/0.00712 d}}{{\text{m}}^{\text{3}}}\);
Award [3] for correct final answer.
Examiners report
In part (a), many candidates failed to score a mark for defining iodine number as they stated it is the amount of iodine, rather than the mass of iodine. The Chemistry guide clearly states in 1.1.2 that amount means the number of moles of a substance. Few candidates could calculate the volume of iodine solution required in part (b). Few recognised that 2 moles of iodine reacts with each mole of linoleic acid. Many tried to use the volume of one mole of gas to find the volume of solution. This is a standard question that is clearly in the Chemistry Guide in B.4.5.
Question
Give the general structural formula for a fat or oil and describe the difference in structure between a saturated and an unsaturated fatty acid.
Explain why unsaturated fats have a lower melting point than saturated fats.
Oils can be hydrogenated. One possible problem is that partial hydrogenation may occur which produces an oil containing trans fatty acids. Explain the structural difference between a cis fatty acid and a trans fatty acid and state one disadvantage of ingesting oils containing trans fatty acids.
Difference:
Disadvantage:
▶️Answer/Explanation
Markscheme
 ;
;
saturated fatty acids contain only single bonds between carbon atoms/C–C whereas unsaturated fatty acids contain at least one double bond between carbon atoms/C=C / OWTTE;
the double (C=C) bond in unsaturated fats causes a “kink” so the molecules cannot pack so closely / OWTTE;
the weaker van der Waals/intermolecular forces between the molecules cause unsaturated fats to have lower melting points;
Difference
in cis the R groups on either side of the C=C point in the same direction and in trans the R groups point in opposite directions / OWTTE /
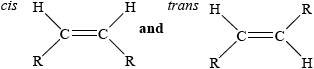 ;
;
Disadvantage
hard to metabolize;
accumulate in fatty tissue;
difficult to excrete;
increase levels of LDL;
not a good source of energy;
Award [1] for any one of the above points.
Examiners report
Few candidates knew the basic structure of a fat or oil in part (a), with the ester linkage frequently missing from the structures drawn. Describing the difference between a saturated and an unsaturated fatty acid was reasonably well answered, although often the strength of the van der Waals‟ forces was not mentioned.
In part (b) most candidates could explain why the melting point of unsaturated fats is lower than that of saturated fats.
Many candidates struggled to adequately describe the structural difference between cis and trans fatty acids in part (c). A simple diagram would have been sufficient. The disadvantage of consuming oils containing trans fatty acids was generally answered well, although some weaker candidates resorted to stating that they were bad for our health. Again, this reflects the difficulties some candidates experience in providing responses with sufficient detail.
Question
Hormones play an important role in the body.
(a) Outline the function and production of hormones in the body.
(b) In many communities there are people who use steroids appropriately, and others who abuse them. Outline one appropriate use and one abuse of steroids.
▶️Answer/Explanation
Markscheme
(a) chemical messenger;
secreted directly into the blood by endocrine glands;
Accept pituitary gland, pancreas, ovaries, testes, thyroid gland and adrenal gland.
(b) build up depleted muscle due to lack of activity;
assist in recuperation from an illness;
stimulation of bone marrow;
treatment of delayed male puberty;
male contraceptive;
treatment of female-to-male gender changes;
treatment of inflammation;
Award [1] for any one use.
muscle/strength build up for an unfair advantage in sport by athletes;
Award [1] for abuse.
Examiners report
Generally well done, though a number of candidates appeared a little confused about where hormones were produced and the fact that they are released directly into the bloodstream. In Part (b) a number of candidates failed to point out that many steroid uses and its abuse are associated with muscle development.
Question
(a) Describe the differences in the structure between the saturated fatty acid \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\) and the unsaturated fatty acid \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{26}}}}{{\text{O}}_{\text{2}}}\).
(b) Describe how \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{26}}}}{{\text{O}}_{\text{2}}}\) can be converted to \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\).
(c) Fatty acids are components of fats and oils.
(i) Describe one advantage of the products formed by hydrogenating fats and oils.
(ii) Describe one disadvantage of the products formed by hydrogenating fats and oils.
▶️Answer/Explanation
Markscheme
(a) unsaturated fatty acid has (3) C=C double bonds;
saturated fatty acid has only single C–C bonds;
unsaturated fatty acid can display cis and trans isomerism / saturated fatty acid cannot display cis and trans isomerism;
saturated fatty acid chains are straighter than unsaturated chains / OWTTE;
(b) hydrogen/ 2 H /hydrogenation;
(high pressure and) high temperature/any temperature in the range of 150 °C to 200 °C /heat;
catalyst/nickel/Ni/platinum/Pt/copper/Cu/zinc/Zn;
(c) (i) semi-solid/solid fat/lipid (with higher melting point);
decreased rate of oxidation/stability increases with increasing saturation;
increased hardness;
control feel and plasticity/stiffness;
hydrogenated vegetable fats are cheaper than animal fats;
(ii) (trans fatty acids can be formed from partial hydrogenation of fats and oils)
(trans fatty acids) are difficult to metabolize/accumulate in fatty tissue/are difficult to excrete;
(trans fatty acids) increase the levels of LDL cholesterol;
(trans fatty acids) are a low quality energy source;
mono- and poly-unsaturated fats are healthier (for the heart) than saturated fats;
Examiners report
This was generally not well done, with many candidates in the first part failing to note the degree of unsaturation as well as not specifying it was carbon-carbon bonds they were referring to. In Part (b) the conditions for hydrogenation reactions appeared not to be well known, though many candidates gained some marks on the advantages and disadvantages of hydrogenated fats, though the latter often lacked the required precision.
Question
Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data Booklet. Determine the mass of iodine, I2, which reacts with 100 g of linoleic acid.
Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value than carbohydrates.
The formula of stearic acid is also given in Table 22 of the Data Booklet. Explain why linoleic acid has a lower melting point compared to stearic acid.
▶️Answer/Explanation
Markscheme
2 mol of iodine reacts with 1 mol of linoleic acid;
\({M_{\text{r}}} = {\text{253.80 g}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) for iodine and \({M_{\text{r}}} = {\text{280.50 g}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) for linoleic acid;
\(\left( {\frac{{(507.60 \times 100.00)}}{{280.50}} = } \right){\text{ }}180.96{\text{ (g)}}/181{\text{ (g)}}\);
Award [3] for correct final answer.
Allow 254 g mol–1 for iodine and 281/280 g\(\,\)mol–1 for linoleic acid.
Award [2 max] for incorrect ratio calculation to give answers such as 90.4, 90.481, 90.7 (g) depending on Mr values used.
less oxidized (compared to carbohydrates) / fewer oxygen atoms (compared to carbohydrates);
C=C’s in linoleic acid cause the chain to be more uneven/kinked;
linoleic acid cannot pack as closely as stearic acid;
intermolecular/van der Waal’s/London/dispersion forces weaker in linoleic acid;
Accept converse argument for stearic acid.
Examiners report
In (a) the vast majority of candidates did not recognise that linoleic acid had two C=C double bonds and hence the ratio n(I2):n(acid) = 2:1. Candidates also made careless errors in calculating the molar mass of either/both iodine or/and linoleic acid.
Part (b) was answered well by about half of the candidates.
In Part(c) many candidates scored both marks, but there were cases which indicated poor preparation of candidates on this rather trivial question which appears so often in examinations.
Question
Determine the number of double bonds in linoleic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\), and linolenic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{30}}}}{{\text{O}}_{\text{2}}}\), and suggest which fatty acid will have a higher iodine number.
Explain why it is important to include the fatty acids linoleic and linolenic acid in a balanced diet.
The partial equation for the enzyme-catalysed hydrolysis of a triglyceride is shown below. Draw the structural formulas of the products A and B.
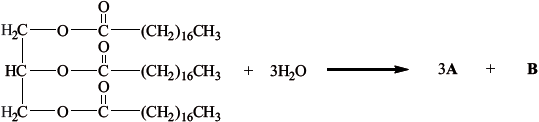
A:
B:
Deduce whether the fatty acid obtained in part (c) will have a higher or lower melting point compared to oleic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{7}}}{\text{CH=CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{7}}}{\text{COOH}}\). Outline your reason.
▶️Answer/Explanation
Markscheme
linoleic has 2 C=C / double bonds and linolenic has 3 C=C / double bonds;
linolenic acid (will have higher iodine value);
Accept linoleic has 3 double bonds and linolenic has 4 double bonds.
essential fatty acids / cannot be synthesized in body;
lowers LDL cholesterol level / lowers risk of heart disease / affects inflammation / conversion to important molecules;
A: \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{{\text{16}}}}{\text{COOH}}\);
B: \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\);
Accept [1 max] if A and B reversed.
Accept full structural formula.
Penalize missing H atoms once only.
higher (melting point);
saturated fatty acids / no unsaturation / no C=C bonds;
Accept appropriate reason such as close packing, no kink in molecule, stronger van der Waals’ forces, larger surface area of contact.
Accept opposite reasons why oleic acid would have a lower mp.
Examiners report
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Question
Fats and vegetable oils are triesters of glycerol with fatty acids. Many of these acids contain 18 carbon atoms. The table shows the relative percentages of various \({{\text{C}}_{{\text{18}}}}\) fatty acid chains in four common fats and oils.
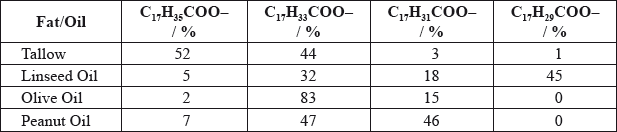
Deduce which fat or oil from the table could best be described as:
saturated
mono-unsaturated
poly-unsaturated.
Hydrogenation can result in the formation of trans fatty acids. Outline the meaning of the term trans fatty acids and explain why their formation is undesirable.
▶️Answer/Explanation
Markscheme
saturated: Tallow;
mono-unsaturated: Olive oil;
poly-unsaturated: Linseed oil;
3 correct award [2], 2 correct award [1], no marks for just one correct.
fats with trans configuration across the double bond;
not easily digested / accumulate in body tissue / increase LDL cholesterol levels;
Do not accept “bad cholesterol”.
Examiners report
Quite a few candidates could correctly identify the relative degree of saturation of the oils, though some thought they had to include all four in their answer. The meaning of shelf life was quite well known, in addition many realised that increasing unsaturation decreased shelf life and could suggest ways of increasing it. The conditions requiredcfor hydrogenation were not well appreciated, especially the need for a catalyst, and few could write any specific details regarding trans-fats apart from the fact there were health concerns regarding these.
Quite a few candidates could correctly identify the relative degree of saturation of the oils, though some thought they had to include all four in their answer. The meaning of shelf life was quite well known, in addition many realised that increasing unsaturation decreased shelf life and could suggest ways of increasing it. The conditions requiredcfor hydrogenation were not well appreciated, especially the need for a catalyst, and few could write any specific details regarding trans-fats apart from the fact there were health concerns regarding these.
Question
Triglycerides are one of three types of lipid found in the human body. The following equation represents the formation of a triglyceride.
X + 3RCOOH \( \rightleftharpoons \) triglyceride + 3Y
Identify the compounds X and Y.
X:
Y:
Draw the structural formula of a triglyceride formed from one molecule each of octanoic acid, lauric acid and stearic acid. The formulas of the acids are shown in Table 22 of the Data Booklet.
Explain whether the triglyceride in part (b) is a solid or a liquid at room temperature.
Identify the type of reaction that occurs during the formation of a triglyceride.
Explain why fats have a higher energy value per mole than carbohydrates.
▶️Answer/Explanation
Markscheme
X is glycerol/propane-1,2,3-triol/ \({\text{C}}{{\text{H}}_2}{\text{(OH)CH(OH)C}}{{\text{H}}_2}{\text{(OH)}}\);
Y is water/ \({{\text{H}}_2}{\text{O}}\);
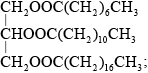
Accept the fatty acids in any order.
solid as contains (three) saturated/straight fatty acid chains;
can pack closer together;
have stronger London/dispersion/van der Waals’ forces between chains;
esterification / condensation;
fats contain less oxygen than carbohydrates / are in a less oxidised state (so more energy is released);
Examiners report
This question which was expected to be fairly straightforward proved to be rather tricky for candidates. In part (a) very few correctly identified glycerol in the formation of a triglyceride.
Drawing the structure of a triglyceride in (b) was challenging for many.
Some candidates explained very well why the triglyceride was a solid at room temperature, but others could only state that it was solid and were unclear of the reasons.
[N/A]
Only the best candidates could explain why fats have a higher energy value per mole than carbohydrates.
Question
Rancidity is the perception of flavours in lipids that our senses perceive as off because of a disagreeable smell, taste, texture or appearance. The processes that create the off-flavours may be hydrolytic rancidity or oxidative rancidity in lipids.
Predict the products of hydrolytic rancidity of fats.
The hydrolysis of milk products is used in the making of cheese. State two conditions which increase the rate of hydrolysis of fats in milk.
▶️Answer/Explanation
Markscheme
(component) fatty acids;
glycerol/propane-1,2,3-triol/ \({\text{C}}{{\text{H}}_2}{\text{(OH)CH(OH)C}}{{\text{H}}_2}{\text{(OH)}}\);
(presence of) enzymes/lipase;
heat;
Examiners report
Some candidates scored well on this question, but there were many weak responses. Candidates needed to relate the packaging of potato crisps to the exclusion of oxygen and light in (a).
Some candidates scored well on this question, but there were many weak responses. Candidates needed to relate the packaging of potato crisps to the exclusion of oxygen and light in (a).
Question
A healthy diet consists of a range of food groups in the right proportions that provide the energy for the body to function, grow and repair itself.
Examples of straight-chain fatty acids include \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\), \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) and \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\).
State the empirical formula and structural features of monosaccharides.
Deduce the structural formula of a triester formed from three long-chain carboxylic acid molecules, RCOOH, and one propane-1,2,3-triol molecule, \({\text{HO–C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)–C}}{{\text{H}}_{\text{2}}}{\text{OH}}\). Identify one of the ester linkages in the structure by drawing a rectangle around it.
Deduce the number of C=C bonds present in one molecule of each fatty acid.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\):
▶️Answer/Explanation
Markscheme
\({\text{C}}{{\text{H}}_2}{\text{O}}\);
Accept (CH2O)n
one carbonyl/C=O and (at least two) hydroxyl/OH groups;
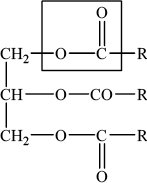
Award [1] for structure that shows unambiguously how the atoms are arranged together.
Award [1] for identifying one of the three ester linkages – must not include R and/or CH2.
\({{\text{C}}_{19}}{{\text{H}}_{39}}{\text{COOH}}\): 0
\({{\text{C}}_{19}}{{\text{H}}_{31}}{\text{COOH}}\): 4
\({{\text{C}}_{19}}{{\text{H}}_{29}}{\text{COOH}}\): 5
All three [2], any two [1], any one [0].
Examiners report
The vast majority of candidates were able to state the empirical formula of monosaccharides, but a good number were not able to state its structural features.
It was surprising to see that only about half could deduce the structure of the triester correctly and identify the ester linkage.
About half of the candidates were able to deduce the number of C=C bonds correctly from the list of fatty acids.
Question
Food shelf life is the time it takes for a particular foodstuff to become unsuitable for eating because it no longer meets customer or regulatory expectations. As a result, in many parts of the world, packaged foods have a date before which they should be consumed.
Rancidity in lipids occurs by hydrolytic and oxidative processes.
State the meaning of the term rancidity as it applies to fats.
Compare the two rancidity processes.
Hydrolytic process:
Oxidative process:
▶️Answer/Explanation
Markscheme
unpleasant/disagreeable smell/taste/texture/appearance;
Hydrolytic process:
lipid converted into glycerol and fatty acid (by hydrolysis of water in presence of enzymes and no C=C present);
Oxidative process:
oxidation of unsaturated fatty acid (chains)/addition of oxygen across C=C/carbon-carbon double bond;
Examiners report
The vast majority of candidates answered correctly.
(b) (i) was poorly answered by the vast majority with only very few candidates scoring some marks here.
Question
A potato chip (crisp) was ignited and the flame was used to heat a test tube containing water.

(i) Calculate the heat required, in kJ, to raise the temperature of the water, using data in the table above and from Table 2 of the Data Booklet.
(ii) Determine the enthalpy of combustion of the potato chip, in \({\text{kJ}}\,{{\text{g}}^{ – 1}}\).
This energy comes mainly from the combustion of triglycerides. State the name of one other type of lipid found in the body and one role, other than energy storage, of this type of lipid.
Name:
Role:
Explain why lipids have a higher energy content than carbohydrates.
▶️Answer/Explanation
Markscheme
(i) \({\text{heat}} = \frac{{4.18 \times 20.0 \times (51.3 – 17.8)}}{{1000}}\);
\( = 2.80{\text{ (kJ)}}\);
(ii) \({\text{enthalpy of combustion}} = \left( {\frac{{2.80}}{{0.421}} = } \right){\text{ }} – 6.65{\text{ (kJ}}\,{{\text{g}}^{ – 1}}{\text{)}}\);
Name:
steroids;
Role:
(sex) hormones;
OR
Name:
phospholipids;
Role:
membranes;
lipids less oxidized/contain less oxygen / carbohydrates partially/more oxidized/contain more oxygen / OWTTE;
Examiners report
This part was generally well answered but there were some cases where 33.5 °C was converted into Kelvin. Many candidates had serious problems with unit conversions and gave the answer as 2800 J or 2800 kJ. Some candidates had correct value for (ii) but lost the mark because of the omission of the negative sign.
Part (b) was well answered.
Very few candidates linked the fact that lipids have higher energy content due to being less oxidized.
Question
Most foods are complex mixtures and many components of them are nutrients.
Identify the types of nutrients A, B and C.
A 
B 
C 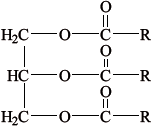
State the names of two types of nutrient other than those shown in part (b).
▶️Answer/Explanation
Markscheme
A: protein / polypeptide/tripeptide;
B: carbohydrate / sugar / monosaccharide / glucose;
C: lipid / triglyceride / vegetable oil / fat;
vitamins;
minerals;
water;
Examiners report
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
Question
Rancidity limits the shelf life of foods containing oils and fats.
Rancidity can occur as a result of two separate processes. State these processes and explain the difference between them.
▶️Answer/Explanation
Markscheme
hydrolytic rancidity and oxidative rancidity;
hydrolytic rancidity involves (reaction with water) breaking ester bond / formation of a fatty acid and glycerol / OWTTE;
oxidative rancidity involves reaction of carbon-carbon double bond/C=C with oxygen / addition reaction with oxygen;
Examiners report
The majority of candidates struggled in part (a) where the difference between hydrolytic and oxidative rancidity was seldom written correctly.
Question
Low-density lipoproteins (LDL) can cause cholesterol to line the walls of the arteries and lead to cardiovascular disease. High-density lipoproteins (HDL) are smaller than low-density lipoproteins.
The formulas of linoleic acid and linolenic acid are given in Table 22 of the Data Booklet. Many vegetable oils are advertised as being a good source of omega-6 fatty acids whereas green leaves are a good source of omega-3 fatty acids.
(i) Identify the major source of low-density lipoproteins.
(ii) State the importance of high-density lipoproteins.
Compare the chemical structures of linoleic acid, an omega-6 fatty acid, and linolenic acid, an omega-3 fatty acid.
▶️Answer/Explanation
Markscheme
(i) saturated fats / fats derived from lauric/myristic/palmitic acid / the liver;
(ii) remove cholesterol from the arteries / transport cholesterol to the liver / protect against heart attack/disease;
both unsaturated fatty acids;
linoleic acid has one less C=C bond/carbon to carbon double bond/is less unsaturated / linolenic acid has one more C=C bond/carbon to carbon double bond/is more unsaturated;
in linoleic the first C=C bond occurs further from the end of hydrocarbon chain / in linolenic the first C=C bond occurs closer from the end of hydrocarbon chain;
both contain 18 carbon atoms;
Examiners report
Most candidates answered part (a) correctly. One respondent stated in the G2 form that ªstudents are not required to know a major source of LDL”, which is a fair comment and will be addressed in future paper editing.
Part (b)(i) proved more challenging and many candidates lost marks resulting from the use of vague terms as “double bonds” rather than “carbon to carbon double bond”. Weaker candidates merely copied the structures from the Data Booklet and even strong candidates often failed to correctly refer the position of the carbon to carbon double bonds.
Question
Fats and oils have some similarities and some differences in their chemical structures.
State two major differences in their structures.
Describe how an oil can be converted into a fat.
Discuss two advantages and two disadvantages of converting oils into fats.
▶️Answer/Explanation
Markscheme
oils contain at least one C=C/carbon to carbon double bond;
oils have fewer carbon atoms in the hydrocarbon chains / OWTTE;
hydrogenation / react with hydrogen (gas);
heat/140−225 °C and metal catalyst/Ni/Zn/Cu/pressure;
Advantages: [2 max]
increases melting points / changes oil to a semi-solid/solid;
decreases rate of oxidation;
increases hardness;
controls feel/plasticity/stiffness;
Disadvantages: [2 max]
the more saturated the less good for the heart / OWTTE;
trans-fatty acids can be formed (through partial hydrogenation);
trans-fatty acids are difficult to metabolize / increase LDL levels / low quality energy source / accumulate in fatty tissue / are difficult to digest/excrete (from the body);
Examiners report
Most candidates compared structural features of fats and oils, but many failed to score as they missed the required specificity of carbon to carbon double bond in (a). A significant number of candidates compared melting points which was not part of the question and very few were able to state the difference in the length of hydrocarbon chains.
Many candidates gave detailed descriptions of the process to score both marks in part (b), but some failed to score the second mark by omitting the need of a catalyst/pressure and/or heat.
Many candidates were able to correctly suggest two advantages but failed to correctly state two disadvantages in part (c). Very often marks were lost as result of poor use of subject specific terms.
Question
There are several types of lipids in the human body. One of these types, triglycerides, might be made of fatty acids with different degrees of saturation.
State one example of each of the following types of fatty acids (refer to Table 22 of the Data Booklet if necessary).
Saturated:
Mono-unsaturated:
Poly-unsaturated:
Describe, by completing the equation below, the condensation of glycerol and the three fatty acids named in (a) to make a triglyceride.
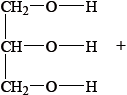
(i) State the names of two other types of lipids present in the human body.
(ii) Compare their composition with that of triglycerides.
▶️Answer/Explanation
Markscheme
Saturated:
octanoic / \({{\text{C}}_7}{{\text{H}}_{15}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\) /
lauric / \({{\text{C}}_{11}}{{\text{H}}_{23}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{10}}{\text{COOH}}\) /
palmitic / \({{\text{C}}_{15}}{{\text{H}}_{31}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{14}}{\text{COOH}}\) /
stearic / \({{\text{C}}_{17}}{{\text{H}}_{35}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{16}}{\text{COOH}}\);
Mono-unsaturated:
oleic / \({{\text{C}}_{17}}{{\text{H}}_{33}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_7}{\text{CH=CH(C}}{{\text{H}}_2}{{\text{)}}_7}{\text{COOH}}\);
Poly-unsaturated:
linoleic / \({{\text{C}}_{17}}{{\text{H}}_{31}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{{\text{(CH=CHC}}{{\text{H}}_2}{\text{)}}_2}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\) /
linolenic / \({{\text{C}}_{17}}{{\text{H}}_{29}}{\text{COOH/C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{(CH}}\)=\({\text{CHC}}{{\text{H}}_2}{{\text{)}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\);
Accept name or formula.
Accept other correct examples of fatty acids.
Accept systematic names instead of trivial names.
This scheme is only one of many possible examples.
the release of three molecules of water;
correct structure of all three ester groups;
Accept more condensed structural formulas.
Ester group must be written correctly, glycerol–OOC–R (not glycerol–COO–R).
Do not penalize for minor mistakes in the hydrocarbon chains or the use of R.
(i) phospholipids and steroids;
Do not accept cholesterol/other specific examples.
(ii) all three types of lipids are (predominantly) hydrophobic/non-polar/consist mostly of hydrocarbon fragments;
triglycerides and (most) phospholipids contain (a fragment of) glycerol;
steroids are (poly)cyclic compounds/contain (several) rings;
phospholipids contain phosphate (group);
triglycerides and phospholipids are esters;
Allow phosphoric acid/phosphorus instead of phosphate in phospholipids.
Allow cholesterol is (poly)cyclic compound/contains (several) rings as ECF from (i).
Examiners report
Most candidates were able to transfer the required information for part (a) correctly from the Data Booklet.
In (b), the mark for three molecules of water was more often gained than the mark for the correct drawing of the ester link which was often the wrong way round (glycerol–COO–R rather than glycerol–OOC–R) – or just wrong.
In (c), cholesterol was most often given in place of steroids. On reflection, composition might not have been the best word to use in (c)(ii), but it seemed to cause candidates little difficulty and the mark-scheme allowed candidates to score well. Candidates need to consider the best way to set out answers asking for a comparison.
Question
Fats are complex molecules derived from fatty acids and glycerol. They are an important part of our diet and have many functions in the body including energy storage.
Identify the main functional group present in
(i) all fats.
(ii) all fatty acids.
▶️Answer/Explanation
Markscheme
(i) ester;
(ii) carboxylic acid / carboxyl;
Accept alkanoic acid.
Accept formulas.
Examiners report
In (a) the ester in fats was less well known than the carboxylic acid in fatty acids.
Question
Many factors affect the shelf life of food products.
Describe the rancidity of fats.
▶️Answer/Explanation
Markscheme
(perception of flavours in lipids due to) disagreeable smell/taste/texture/appearance / OWTTE;
Examiners report
Many candidates missed the command term used in part (c) and failed to describe the rancid food.
Question
Lipids play a significant role in human nutrition and have many important biological functions. The triglycerides are one type of lipid.
Table 22 of the Data Booklet shows the formulas of some fatty acids.
Olive oil contains a triglyceride (glyceryl trioleate) which, on hydrolysis, yields propane-1,2,3-triol (glycerol) and oleic acid.
Deduce the equation for this reaction. You may use the letter R to represent the hydrocarbon chains.
Calculate the iodine number for oleic acid (\({M_{\text{r}}}\) of oleic acid \( = 282.52\)).
Linoleic acid and stearic acid have similar molecular masses. Explain why linoleic acid has a much lower melting point than stearic acid.
Linoleic acid and linolenic acid are classed as essential fatty acids. State the importance of these fatty acids in the human diet.
▶️Answer/Explanation
Markscheme
correct structure for triglyceride;
correct structures for products;
correct balancing;
Mark for balancing can only be awarded if reactants and products are correct.
Accept more condensed structural formula, but ester group must be the correct
way round (glycerol–OOC–R or glycerol–O–CO–R, not glycerol–COO–R).
Do not penalize minor errors in the hydrocarbon chain or in the use of R.
100 g of oleic acid reacts with \(\frac{{{\text{253.8}} \times {\text{100}}}}{{{\text{282.52}}}}{\text{ (g)}}\) of \({{\text{I}}_{\text{2}}}\);
Do not penalize use of integer values for \({M_r}\).
hence iodine number is 89.9;
Accept answers between 89.7 and 90.
Award [2] for for correct answer.
Award [1] for an iodine number between 44.8 and 45.0 if monatomic iodine is assumed.
Award [1] for an iodine number between 0.897 and 0.900 if 1 g of oleic acid assumed.
Award [1] for an iodine number between 111 and 112 – mass of I2 reacting with 100 g of oleic acid.
in linoleic acid, presence of C=C/double bond/unsaturation prevents close packing/leads to kinks/bends in chain;
Do not allow mark without reference to C=C/double bond/unsaturation.
hence weaker van der Waals’/London/dispersion forces between molecules;
Accept opposite statements for stearic acid but must point out that this is because it does not have a C=C/double bond/unsaturation.
cannot be synthesized by body;
Accept specific uses such as lowers LDL cholesterol level, increases HDL
cholesterol level or lowers risk of heart disease.
Do not accept just “lowers cholesterol level”.
Examiners report
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
Question
The following products result from the hydrolysis of a triglyceride.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{27}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\)
Draw a possible structure for the triglyceride.
State the other reactant and one essential condition that would favour this hydrolysis reaction in the body.
Identify which product is polyunsaturated, and outline why foods containing this type of fatty acid are important for health.
People who live in very cold regions need a diet with a higher ratio of fat to carbohydrate than people who live in warmer climates. Suggest why this is the case.
▶️Answer/Explanation
Markscheme
 ;
;
Accept alternative orders for the hydrocarbon tails.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\) and enzyme/biological catalyst/lipase;
Accept acidic/alkaline/basic condition instead of water.
Do not award mark for lipase alone without water/ H2O.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\);
they lower level of LDL cholesterol/low-density lipoproteins / reduce (the risk of) heart disease;
Allow comparison with saturated fats with explanation.
fats provide more energy (per kg) than carbohydrates;
Do not allow “fat is an insulator”.
Examiners report
It was clearly a challenge for candidates to reconstruct the triglyceride and in (b) conditions for the formation (rather than hydrolysis) of the triglyceride were given. Careful reading of questions is important. If water was given in (b), the enzyme was omitted or replaced by “heat”. In (c), the unsaturated product was often given in place of the polyunsaturated and only cholesterol was discussed rather than LDL cholesterol. The common error in (d) was to state that lipids “provide better insulation”.
Question
Stearic acid, oleic acid and linolenic acid are all fatty acids that contain 18 carbon atoms. Their structures are given in Table 22 of the Data Booklet.
Partial hydrogenation of linolenic acid may lead to a product known as a trans fatty acid.
Explain which acid has the highest melting point.
Discuss two potential problems or health concerns associated with trans fatty acids.
▶️Answer/Explanation
Markscheme
stearic acid;
saturated molecule / more closely packed / greater surface area (of contact) / not “kinked”;
more/stronger van der Waals’ forces;
Accept intermolecular/London/dispersion forces instead of van der Waals’ forces.
trans fats harder to metabolize / accumulate in tissue;
increase levels of LDL cholesterol/low-density lipoprotein / increase risk of heart disease;
low-quality energy source;
Examiners report
Even though the correct acid was often not identified, explanations for the highest melting point often gained full credit in (a). Disappointingly few candidates were able to write a correct equation in (b) (with the addition of three moles of \({{\text{H}}_{\text{2}}}\) (g)) although the conditions were usually correct. In (c), many were able to show a trans orientation – but didn’t use a fatty acid – and many did not score both marks in (ii).
Even though the correct acid was often not identified, explanations for the highest melting point often gained full credit in (a). Disappointingly few candidates were able to write a correct equation in (b) (with the addition of three moles of \({{\text{H}}_{\text{2}}}\) (g)) although the conditions were usually correct. In (c), many were able to show a trans orientation – but didn’t use a fatty acid – and many did not score both marks in (ii).
Question
The formula of linoleic acid is given in Table 22 of the Data Booklet.
Identify the structural formula of the triglyceride formed when three molecules of linoleic acid react with one molecule of glycerol (propane-1,2,3-triol), \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
State the other product formed during this reaction.
Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a solid at room temperature.
Describe how the triglyceride formed from linoleic acid and glycerol could be converted into a saturated fat and give any necessary conditions.
Other than the fact that it is a solid at room temperature, discuss two advantages and two disadvantages of a saturated fat compared to an unsaturated fat or oil.
Advantages:
Disadvantages:
▶️Answer/Explanation
Markscheme
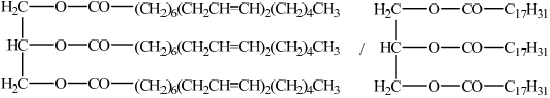 ;
;
Do not accept R for C17H31.
Penalize for incorrect bond connectivity.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
double bonds cause a “kink” in the hydrocarbon chain / unsaturated hydrocarbon chains cannot pack so closely together (as saturated);
attractive forces/London/dispersion/van der Waals/vdW/LDF/ /instantaneous/temporary induced dipole-induced dipole forces between the molecules are weaker / less energy required to overcome the attraction between the molecules;
(d) addition of hydrogen/\({{\text{H}}_{\text{2}}}\) / hydrogenation;
heat and catalyst/Zn/Cu/Ni/Pd/Pt;
Accept any temperature in range 140–225 °C.
Advantages:
decreases rate of oxidation / makes it more stable / slows rancidification / has longer shelf life;
greater energy released per gram / OWTTE;
controls hardness/plasticity/stiffness;
Disadvantages:
increase risk of heart disease / increase low-density/LDL cholesterol;
does not contain essential/omega-3/omega-6 fatty acids;
hydrogenated fats might contain trans-fatty acids;
Examiners report
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Question
Lipids are a group of naturally occurring largely non-polar biomolecules. The term iodine number is often used to characterize particular lipids.
(i) Define the term iodine number.
(ii) A sample containing \(1.12 \times {10^{ – 2}}\) mol of fatty acid was found to react with 8.50 g of iodine, \({{\text{I}}_{\text{2}}}\). Calculate the number of carbon-carbon double bonds present in the fatty acid, showing your working.
(i) Draw the structure of glycerol (propane-1,2,3-triol).
(ii) Glycerol can react with three molecules of lauric acid to form a triglyceride.
The structure of lauric acid is given in Table 22 of the Data Booklet. State the name of the functional group of the triglyceride and identify the other product formed.
Name of functional group of triglyceride:
Other product formed:
The hydrolysis of tristearin, whose structure is shown below, can be catalysed by the enzyme lipase.
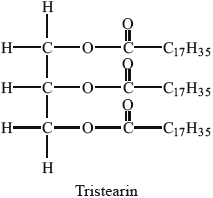
Successive hydrolysis of tristearin results in the formation of distearin and monostearin.
Deduce the structure of the diglyceride, distearin, and state the name of the other product formed from this reaction.
Structure of diglyceride, distearin:
Name of other product:
Explain why the metabolism of fats produces much more energy per gram than that of carbohydrates.
▶️Answer/Explanation
Markscheme
(i) mass (in g) of \({{\text{I}}_{\text{2}}}\) reacting with 100 g of fat/oil/substance/lipid;
Allow amount/number of mol of I2 reacting with 1 mol of fat/oil/substance/lipid.
(ii) \(\frac{{8.50}}{{253.4}}/\frac{{8.50}}{{254}}/3.35 \times {10^{ – 2}}{\text{ (mol)}}\);
\(\left( {\frac{{3.35 \times {{10}^{ – 2}}}}{{1.12 \times {{10}^{ – 2}}}}} \right) = 3\) (C=C);
OR
\(\frac{{8.50}}{{1.12}} \times {10^{ – 2}}/759\) (g \({{\text{I}}_{\text{2}}}\) react with one mol of fatty acid);
\(\left( {\frac{{759}}{{254}}} \right) = 3\) (C=C);
M2 can only be awarded if M1 is correct.
(i) 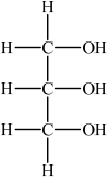 ;
;
Accept any correct representation.
(ii) Name of functional group of triglyceride:
ester
Allow triester.
Do not allow –COO–.
and
Other product formed:
water/\({{\text{H}}_{\text{2}}}{\text{O}}\)
Structure of diglyceride, distearin:
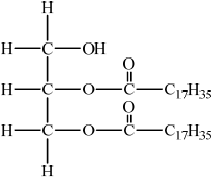 ;
;
Accept a structure with OH in middle also.
Name of other product:
stearic acid/octadecanoic acid / stearate/octadecanoate;
Name required.
Do not allow stearin.
fats have fewer oxygens than carbohydrates (of same molar mass) / fats less oxidized;
Allow converse statements for carbohydrates.
a larger change in carbon’s oxidation number occurs when fats are oxidized / more energy is used in breaking the bonds in carbohydrates than the bonds in fats;
Examiners report
(i) About half of the candidates could define iodine number correctly. Others had the correct idea but gave imprecise answers.
(ii) About half of the candidates obtained the number of moles of iodine that reacted and gained one mark. A smaller number of candidates were able to calculate the number of double bonds in the fatty acid.
(i) Almost all candidates were able to draw the structure of glycerol.
(ii) About half of the candidates recognized the functional group in a triglyceride as an ester, and more than half of the candidates recognized water as the other product formed.
This was a discriminating question. Only a few candidates were able to deduce the structure of the diglyceride and even fewer recognized the other product as stearic acid.
Many candidates obtained a mark for stating that fats contain less oxygen or are less oxidized than carbohydrates, but were unable to score the second mark.
Question
Food chemistry and nutritional science are two important scientific fields to which the general public relate.
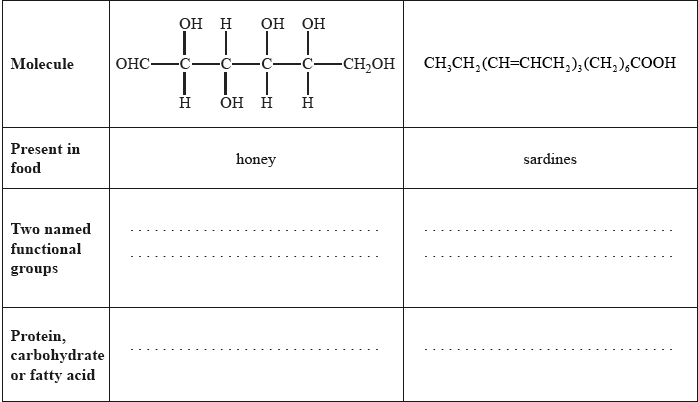
State two named functional groups present in each of the following molecules found in two different food products (honey and sardines). Identify each molecule as a protein, a carbohydrate or a fatty acid.
Butter is an example of a saturated fat and olive oil is an example of an unsaturated fat. Describe the main structural difference between these two types of fat.
Linoleic acid, whose structure is given in Table 22 of the Data Booklet, is present in peanut oil. The oil can be converted to a semi-solid using hydrogen gas. Predict the structural formula of the compound formed from the partial hydrogenation reaction of linoleic acid, and state a suitable catalyst for this reaction.
Structural formula:
Catalyst:
Partial hydrogenation can sometimes produce trans fats. Suggest why trans fats are considered unhealthy.
Olestra, with one of its structures shown below, has been used to prepare snacks such as crisps (potato chips). Deduce the type of compound that can undergo an esterification reaction involving carboxylic acid to produce olestra.
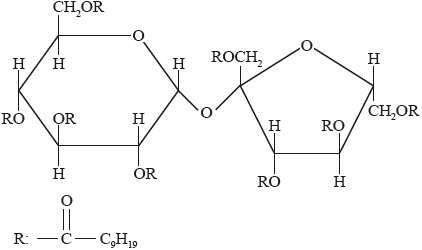
Olestra
▶️Answer/Explanation
Markscheme
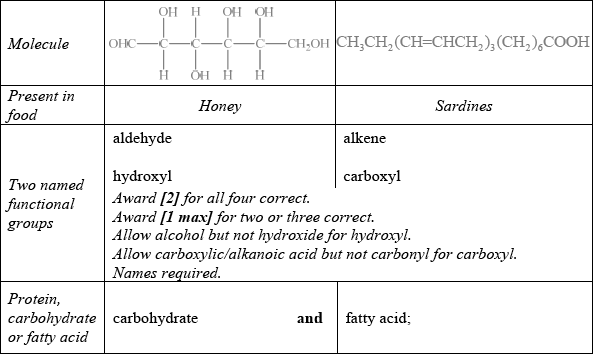
Saturated fat: no carbon-carbon double bonds/no C=C/all single carbon-carbon bonds/ all C–C and Unsaturated fat: carbon-carbon double bonds/C=C/alkene groups;
Mention of carbon-carbon or alkene necessary for mark.
Structural formula:
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CH}}\)=\({\text{CH(C}}{{\text{H}}_2}{{\text{)}}_7}{\text{COOH}}\) /
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{CH}}\)=\({\text{CHC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\);
Catalyst: nickel/Ni / palladium/Pd / platinum/Pt / copper/Cu / zinc/Zn;
decrease (blood) levels of HDL/high-density lipoprotein cholesterol (which protects from heart disease) / increase levels of LDL/low-density lipoprotein cholesterol (increasing risk of heart disease) / less easily digested/metabolized / leads to blocked arteries;
carbohydrate / disaccharide;
Allow sucrose / sugar.
Examiners report
Well answered with most candidates gaining two marks. The most common mistake was failing to recognize OHC– as an aldehyde.
The majority of candidates distinguished between saturated and unsaturated fats correctly.
The catalyst was usually well suggested but the structural formula was only provided by the strongest candidates. Some candidates gave the saturated product not taking note of the word “partial” in the question.
Another question better answered than in previous sessions. Many candidates related trans fats to LDL cholesterol.
This was a discriminating question. Only a few candidates recognized the compound as a carbohydrate.
Question
Define the term iodine number.
Diets that are high in omega-3 fatty acids are recommended as healthy for the heart. Eicosapentaenoic acid \(({M_{\text{r}}} = 302)\) is a common omega-3 fatty acid found in fish oils. Calculate the number of carbon-carbon double bonds in one molecule of this acid if 3.02 g of this acid reacts with 12.7 g of \({{\text{I}}_{\text{2}}}{\text{ }}({M_{\text{r}}} = 254)\).
▶️Answer/Explanation
Markscheme
mass (in g) of I2/iodine reacting with 100 g of fat/oil/substance/lipid;
Allow “grams of I2” instead of “mass (in g) of I2”.
Allow amount/number of mol of I2 reacting with 1 mol of fat/oil/substance/lipid.
Do not accept mass number for mass.
\(\left( {\frac{{3.02}}{{302}} = } \right){\text{ }}0.0100{\text{ (mol)}}\) acid;
\(\left( {\frac{{12.7}}{{254}} = } \right){\text{ }}0.0500{\text{ (mol) }}{{\text{I}}_2}\);
\(\left( {\frac{{0.0500}}{{0.0100}} = } \right){\text{ }}5\) (carbon-carbon double bonds);
Award [1 max] for 5 if no working shown.
Examiners report
The better candidates gave the correct definition of iodine number, namely the mass in grams of iodine reacting with 100 g of fat. This is the precise definition of iodine number that should be emphasised to candidates in the teaching programme. Some candidates incorrectly stated amount in grams instead of mass in grams of iodine, which showed poor understanding of the inherent difference between mass, measured in grams, and amount, measured in moles. The iodine number calculation on calculating the number of C=C in eicosapentaenoic acid was well answered and full marks were typically scored.
The better candidates gave the correct definition of iodine number, namely the mass in grams of iodine reacting with 100 g of fat. This is the precise definition of iodine number that should be emphasised to candidates in the teaching programme. Some candidates incorrectly stated amount in grams instead of mass in grams of iodine, which showed poor understanding of the inherent difference between mass, measured in grams, and amount, measured in moles. The iodine number calculation on calculating the number of C=C in eicosapentaenoic acid was well answered and full marks were typically scored.
Question
Most foods contain nutrients.
Triglycerides are formed by the reaction of propane-1,2,3-triol (glycerol) with fatty acids.
(i) State the name of the functional group circled in the triglyceride.
(ii) Identify the other product of the reaction.
(i) State the difference in structure between the fatty acids found in an oil and those in a fat.
(ii) Comment on the relative stability of oils and fats and state the names of two possible types of degradation reaction.
▶️Answer/Explanation
Markscheme
(i) ester;
(ii) water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
(i) (fatty acids in) oils are unsaturated/contain (many) C=C/carbon-carbon double bonds / (fatty acids in) fats are (mostly) saturated/contain no/few/fewer (than oils) C=C /carbon-carbon double bonds;
(ii) C=C bonds degrade/oxidize more rapidly / oils become rancid more rapidly / fats are more stable;
Award [1 max] for any two of:
auto-oxidation;
Allow oxidative rancidity.
Do not accept “reaction with oxygen” (name required).
photo-oxidation;
Do not accept light.
microbial rancidity;
hydrolysis;
Allow hydrolytic rancidity.
Do not accept “addition of water” (name required).
Do not accept hydrogenation (since not a degradation reaction).
Examiners report
In (b) (i), the better candidates stated ester. The weaker candidates incorrectly suggested either alcohol or carboxylic acid. Water was universally known in (ii).
(c) was well answered though some did not score full marks by suggesting that hydrogenation is a degradation reaction which is incorrect.
Question
Lipids are a diverse group of compounds found in the body.
Cholesterol is one of the most important steroids. It plays an essential role in metabolism and is the starting point for the synthesis of many important chemicals in the body.
Compare the structures and polarities of fats and phospholipids, giving one similarity and one difference in structure and one difference in polarity.
Similarity in structure:
Difference in structure:
Difference in polarity:
Vitamin D is produced from cholesterol. The structures of both molecules are given in table 21 of the data booklet. Outline one structural difference between the molecules.
Distinguish between HDL and LDL cholesterol in terms of their composition and their effect on health.
Composition:
One effect on health:
▶️Answer/Explanation
Markscheme
Similarity in structure:
both are (tri)esters / both made from glycerol/propane-1,2,3-triol/HOCH2CH(OH)CH2OH;
Difference in structure:
phospholipids have phosphate group/phosphorus and fats are triglycerides/made from three fatty/carboxylic acids / one fatty/carboxylic acid (in fat) replaced by phosphate in phospholipid;
Difference in polarity:
phospholipids are more polar / phospholipids have hydrophilic (heads/section/part/end) / fats are less polar/non-polar / fats are hydrophobic ;
(two) more carbon−carbon double bonds/alkenyl groups in vitamin D;
Accept alkene for alkenyl.
extra hexagon/6-membered ring in cholesterol / more fused rings in cholesterol / four fused rings in cholesterol and two fused rings in vitamin D;
Accept “(some) conjugation in vitamin D / (some) alternating C=C and C−C bonds in vitamin D”.
Accept “cholesterol has a steroid backbone/structure but vitamin D does not”.
Composition:
HDL has more protein and less cholesterol/fat/lipid (and vice-versa);
Accept “HDL has more protein and LDL has more cholesterol (and vice-versa)”.
Accept “HDL has higher phospholipid content compared to LDL (and vice-versa)”.
Accept “HDL particles are smaller than LDL particles (and vice-versa)” but do not penalize if “molecules” are used instead of “particles”.
One effect on health:
cardiovascular problems/increased risk of heart disease/obesity/atherosclerosis/blocked arteries from high ratio of LDL to HDL;
Accept “from (high) LDL” instead of “from high ratio of LDL to HDL”.
Accept “can result in a heart attack/stroke from high ratio of LDL to HDL”.
Accept “large amounts of HDL in blood correlate with good health / OWTTE”.
Reference must be made to LDL or HDL.
Examiners report
About half the candidates seemed familiar with the structures of fats and phospholipids, but only a few gave the detailed answers expected by the markscheme. Some of the answers were far too general such as stating “both contain C, H and O” for the similarity in structure.
About a third of the candidates scored the mark by stating an accurate structural difference between vitamin D and cholesterol. Some answers were not specific enough about the numbers and types of rings.
Only a few candidates were able to distinguish between the composition of HDL and LDL cholesterol, but the majority of candidates understood their effects on health well and gained the second mark. There was a comment on a G2 form that this question went beyond the syllabus, however, the question is covered in part B.4.2 (“outline the difference between HDL and LDL cholesterol”) and has also appeared in a past paper.
Question
F and G are two synthetic hormones. The structures of some natural hormones are given in table 21 of the data booklet.
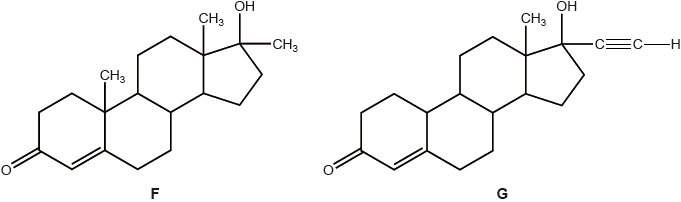
A number of famous athletes have been banned from competition for using hormone F.
Explain, with reference to its structure, why hormone F improves performance.
▶️Answer/Explanation
Markscheme
structure/function similar to testosterone;
causes increased rate of protein synthesis/tissue/muscle building/increase in muscle mass / OWTTE;
Accept “anabolic” for M2.
Examiners report
Generally well-answered. Some candidates thought hormone F was testosterone.
Question
Although people may consume a large amount of food, they may still not consume sufficient nutrients.
Describe one similarity and one difference between the structure of a saturated and an unsaturated fat.
Similarity:
Difference:
▶️Answer/Explanation
Markscheme
Similarity:
both are (tri)esters / both made from glycerol/propane-1,2,3-triol/HOCH2CH(OH)CH2OH / both are triglycerides;
Difference:
unsaturated fats have C=C/carbon−carbon double bond / saturated fats have no C=C/carbon−carbon double bonds;
Examiners report
Many candidates are still forgetting to specify that the difference is in carbon-carbon double bonds. This is important as both fats contain carbon-oxygen double bonds.
Question
Explain, giving their names, the two types of reaction by which foods may become rancid.
Reaction 1:
Reaction 2:
▶️Answer/Explanation
Markscheme
Reaction 1: hydrolytic (rancidity)/hydrolysis and
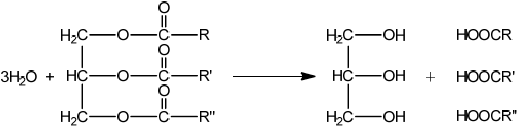
OR
hydrolytic (rancidity) and hydrolysis of ester links/breaking down of lipid/fat to glycerol/propane-1,2,3-triol and fatty/carboxylic acids;
Reaction 2: oxidative (rancidity)/oxidation and addition of O2 across C=C/carbon−carbon double bonds / oxidation of C=C/carbon−carbon double bonds;
Do not penalize omission of “carbon−carbon” if already penalized in F.17(b)(i).
Award [1 max] for “hydrolytic (rancidity)/hydrolysis” and “oxidative (rancidity)/oxidation” only.
Examiners report
Some candidates gained a mark for naming both processes by which food becomes rancid. However, candidates rarely explained the reactions correctly.
Question
Linolenic acid (omega-3 fatty acid) is an essential fatty acid.
List two benefits of linolenic acid to humans.
Calculate the iodine number for linolenic acid, C17H29COOH \(({M_{\text{r}}} = 278.48)\).
The condensed structural formula of linolenic acid is given in table 22 of the data booklet.
▶️Answer/Explanation
Markscheme
assists brain function;
enables normal growth/development;
involved in synthesis of prostaglandins;
reduces (risk of) heart disease/blockage of arteries;
lowers blood pressure;
reduces LDL cholesterol;
increases HDL cholesterol;
Do not accept bad/good instead of LDL/HDL.
\({{\text{n}}_{{\text{iodine}}}} = {\text{3}}{{\text{n}}_{{\text{linolenic acid}}}}\);
\({\text{iodine number}}\left( {\frac{{3 \times 100 \times 253.8}}{{278.48}}} \right) = 273{\text{ (g)}}\);
Award [2] for final correct answer.
If an alternative definition, in terms of moles/C=C bonds is given, award [1 max] for iodine number = 3.
Examiners report
The benefits of linolenic acid were reasonably well known especially its effect on lowering LDL cholesterol and many candidates scored full marks for this part of the question although in some cases, one of the marks was not achieved due to LDL not being specified. The definition of iodine number sometimes proved challenging with some confusion of mass and moles of iodine. The expected definition of the iodine number is the mass in grams of iodine reacting with 100g of oil. Overall, students did find the calculation of the iodine number challenging.
Question
Cholesterol is in our diet and is produced in the body. It is used to produce steroid hormones and is important in membrane structures.
Aldosterone is one of the steroid hormones produced in the body from cholesterol.
The structure of cholesterol is shown in table 21 of the data booklet. Compare the structures of cholesterol and aldosterone by naming two functional groups present in both and two functional groups present only in aldosterone.
▶️Answer/Explanation
Markscheme
Present in both:
alkenyl and hydroxyl;
Accept alkene and alcohol/hydroxy but not hydroxide.
Present only in aldosterone:
carbonyl and aldehyde;
Accept ketone (for carbonyl).
Accept primary hydroxyl/alcohol.
Award [1 max] if correct formulas, rather than names, given for both.
Examiners report
Many students were able to name the functional groups in aldosterone but often were not able to identify both of the functional groups present in both steroid hormones. There were a fair number of references to the ‘hydroxide’ group instead of hydroxyl. The endocrine gland was often not correctly identified although the function of progesterone of testosterone was often answered correctly albeit not always precisely.
Question
Olive oil is a complex mixture of triglycerides, some of which are derived from oleic acid.
State the name of the compound which combines with fatty acids to form triglycerides.
Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
▶️Answer/Explanation
Markscheme
glycerol / propan-1,2,3-triol;
Accept minor errors in naming, such as propane-1,2,3-triol.
hard to metabolize / accumulates in fatty tissue / difficult to excrete;
increases levels of LDL cholesterol / increases risk of heart disease;
Do not accept “increases level of bad cholesterol”.
Examiners report
Most students correctly identified glycerol as the compound which combines with fatty acids to form triglycerides. Students often described the difference between cis and trans isomer but did not always refer to the packing of the molecules. Many candidates knew one effect on health of consuming trans fatty acids with answers often referring to the greater risk of heart disease. Common incorrect effects on health of consuming trans fatty acids were that they were “difficult to break down” or “difficult to digest”. These statements are not synonymous with trans fatty acids being “hard to metabolize”.
Question
Dehydroepiandrosterone (DHEA) is a substance banned under the World Anti-Doping Code.
Steroid abuse has certain health hazards, some general, some specific to males and some specific to females. Identify one health hazard in each category.
General Hazard:
Male Hazard:
Female Hazard:
(i) State the name of the functional group circled in the DHEA molecule shown below.
(ii) Identify the characteristic of this structure that classifies it as a steroid.
The production of banned steroids has ethical implications. Suggest a reason why steroid research might be supported.
▶️Answer/Explanation
Markscheme
General hazards:
acne
OR
weight gain
OR
liver/kidney damage
OR
stunted growth
OR
disruption of puberty
OR
increased aggressiveness
OR
increased risk of heart disease/atherosclerosis/heart attacks/strokes√
General hazards:
Accept heart problems.
Male hazards:
feminization/breast «tissue» development
OR
shrinking of the testes/testicles
OR
reduction in sperm production
OR
impotence
Male hazards:
Accept baldness.
Female hazards:
decreased breast development
OR
masculinisation
OR
infertility/abnormal menstrual cycles
OR
birth defects/altered fetus development
(i)
alkenyl/ethanylylidene
(ii)
four-ring «steroidal» backbone
OR
fused ring structure
OR
three 6-membered rings AND a 5-membered ring
Award [1] for a sketch of the steroidal backbone.
medical uses of steroids «under physician supervision»
OR
detection of banned substances can be improved
Accept any specific medical use.
Accept answers such as “their effects «either positive or negative» are better understood”.
Question
Lipids are an important part of the human diet.
Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed in this reaction and the name of the other product.
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
▶️Answer/Explanation
Markscheme
Name of the chemical link: ester/ethoxycarbonyl
AND
Name of the other product: water
Do not accept formulas.
Do not accept “esterification”
i
coconut oil AND lowest «percentage of» unsaturated fatty acids
OR
coconut oil AND smallest number of C=C bonds
OR
coconut oil AND highest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
ii
soybean oil AND highest «percentage of» polyunsaturated fatty acids
OR
soybean oil AND greatest number of C=C bonds
OR
soybean oil AND lowest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
iii
Beef fat: «P/S = \(\frac{3}{{59}}\) = » 0.05
AND
Soybean oil: «P/S = \(\frac{{50 + 8}}{{14}}\) =» 4.1
iv
«higher proportion of» polyunsaturated fatty acids decrease risk of atherosclerosis/heart disease/cardiovascular disease/CVD
OR
«higher proportion of» polyunsaturated fatty acids which are less likely to be deposited on the walls of arteries «than saturated fatty acids»
Accept converse arguments.
Accept correct arguments in terms of HDL and LDL but not in terms of “good” and “bad” cholesterol.
Accept “fats” for “fatty acids”.
v
Any two of:
cotton seed oil has «a higher proportion of» longer chain/greater molar mass fatty acids
molecules of cotton seed oil have greater surface area/have higher electron density
Accept “molecules of cotton seed oil are packed more closely/have more regular structure” for M2.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces between chains in cotton seed oil
Accept converse arguments.
Accept “fats” for “fatty acids”.
Question
Lipids and carbohydrates contain the same elements but have different properties.
List the building blocks of triglycerides and carbohydrates.
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16.
▶️Answer/Explanation
Markscheme
Triglycerides:
organic acid/fatty acid and glycerol/propane-1,2,3-triol
AND
Carbohydrates:
monosaccharides
Accept simple sugars.
[1 mark]
«water/aqueous solubility depends on forming many» H-bonds with water
raffinose has many hydroxyl/O–H/oxygen atoms/O «and forms many H-bonds» AND linoleic acid has few/one hydroxyl/O–H/oxygen atom/O/carboxyl group/ COOH/is largely non-polar «and cannot form many H-bonds»
Accept statement which implies comparison.
[2 marks]
«base» hydrolysis/saponification
OR
«produces glycerol and» soap/salt of the «fatty» acid
«products are» water soluble «and drain away»
Accept condensed formulas.
Accept non-balanced equation.
Accept “RCOONa”.
[2 marks]
linoleic acid/C18H32O2 combustion/oxidation more exothermic «per mol»
linoleic acid/C18H32O2 has lower proportion/number of O atoms
OR
linoleic acid/C18H32O2 is less oxidized
Accept converse arguments.
[2 marks]
Question
Sunflower oil contains stearic, oleic and linoleic fatty acids. The structural formulas of these acids are given in section 34 of the data booklet.
Explain which one of these fatty acids has the highest boiling point.
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
▶️Answer/Explanation
Markscheme
stearic acid AND chain has no kinks/more regular structure
OR
stearic acid AND it has straight chain
OR
stearic acid AND no C=C/carbon to carbon double bonds
OR
stearic acid AND saturated
OR
stearic acid AND chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “stearic acid AND greater surface area/electron density”.
M2 can only be scored if stearic acid is correctly identified.
Accept “stronger intermolecular/van der Waals’/vdW forces”.
[2 marks]
«n(I2) = 0.123 dm3 x 0.500 mol\(\,\)dm–3 =» 0.0615 «mol»
«m(I2) = 0.0615 mol x 253.8 g\(\,\)mol–1 =» 15.6 «g»
«iodine number \( = \frac{{15.6{\text{ g}} \times 100}}{{10.0{\text{ g}}}}\)» = 156
Award [3] for correct final answer.
Iodine number must be a whole number.
Award [2 max] for 78.
[3 marks]
Question
A chemical reaction occurs when a phospholipid is heated with excess sodium hydroxide.
Glycerol is one product of the reaction. Identify the two other organic products.
Identify the type of reaction which occurs.
▶️Answer/Explanation
Markscheme
C17H31COONa
[(CH3)3NCH2CH2OH]OH
Accept “NaC17H31COO”.
Accept “(CH3)3N+CH2CH2OH OR [(CH3)3NCH2CH2OH]+” if positive charge is shown.
Accept suitable names (eg, sodium linoleate, choline hydroxide etc.) OR correct molecular formulas.
[2 marks]
hydrolysis
Accept “nucleophilic substitution/displacement / SN/SN2 /saponification”.
Do not accept “acid hydrolysis”.
[1 mark]
Question
Consider the following lipid and carbohydrate.
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
▶️Answer/Explanation
Markscheme
C9H16O
ratio of oxygen to carbon in linoleic acid lower
OR
linoleic acid less oxidized
OR
linoleic acid more reduced
Accept “«average» oxidation state of carbon in linoleic acid is lower”.
«electrophilic» addition/AE
OR
oxidation–reduction/redox
«\(\frac{{1.24\,{\text{g}}}}{{280.50\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\) =» 0.00442 «mol»
0.00884 mol of C=C
OR
ratio of linoleic acid : iodine = 1:2
«volume of I2 solution = \(\frac{{0.00884\,{\text{mol}}}}{{0.300\,{\text{mol}}\,{\text{d}}{{\text{m}}^{ – 3}}}}\) =» 0.0295 «dm3» / 29.5 «cm3»
Award [3] for correct final answer.
Any two of:
increases «ratio of» HDL «to LDL» cholesterol
OR
decreases LDL cholesterol «level»
removes plaque from/unblocks arteries
OR
decreases risk of heart disease
decreases risk of stroke «in the brain»
Accept “essential fatty acid”.
Do not accept “bad cholesterol” for “LDL cholesterol” OR “good cholesterol” for “HDL cholesterol”.
Do not accept general answers such as “source of energy” OR “forms triglycerides” OR “regulates permeability of cell membranes” etc.
[Max 2 Marks]
Question
Saturated lipids found in butter and unsaturated lipids found in fish oil readily become rancid.
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
State one factor that increases the rate at which saturated lipids become rancid.
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
▶️Answer/Explanation
Markscheme
hydrolytic «rancidity»
ester group
Accept a formula for ester group.
[2 marks]
«presence of» moisture/water
OR
«increase in» temperature
OR
«presence of» enzymes/bacteria/fungi/mould
OR
low pH/«presence of» acid
Accept “heat”.
[1 mark]
«stearic acid» straight chain/chain has no kinks/more regular structure
OR
«stearic acid» saturated/no «carbon–carbon» double bonds
«stearic acid» chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “«stearic acid» greater surface area/electron density”.
[3 marks]
lowers risk of heart disease/atherosclerosis
OR
lowers LDL cholesterol
OR
increases HDL cholesterol
OR
aids brain/neurological development «in children»
OR
relieves rheumatoid arthritis
[1 mark]
soluble AND non-polar hydrocarbon chain
Accept as reasons “«predominantly» non-polar” OR “long hydrocarbon chain”.
[1 mark]
not biodegradable
OR
stored/accumulate in fat
biomagnification occurs
OR
concentration increases along food chain
Accept “stored/accumulate in bodies of prey/animals eaten”.
Accept “not excreted”.
[2 marks]
add starch/cellulose/carbohydrates/additives/catalysts «to plastic during manufacture to allow digestion by micro-organisms»
OR
replace traditional plastics with polylactic acid/PLA-based ones
OR
blend traditional and polylactic acid/PLA-based plastics
Accept reference to biodegradable plastics other than PLA; for example polyhydroxyalkanoates (PHA), poly(butylene succinate) (PBS), polybutylene adipate terephthalate (PBAT) and polycaprolactone (PCL).
[1 mark]
Question
Lipids provide energy and are an important part of a balanced diet.
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
Explain why lipids provide more energy than carbohydrates and proteins.
▶️Answer/Explanation
Markscheme
Type of reaction:
condensation
OR
esterification/triesterification
OR
nucleophilic substitution/nucleophilic displacement/SN2
By-product:
water/H2O
Do not accept just “substitution/displacement”.
[2 marks]
ALTERNATIVE 1
«\(\frac{{334}}{{253.8}}\) =» 1.32 AND «\(\frac{{100}}{{304.5}}\) =» 0.328
«\(\frac{{1.32}}{{0.328}}\) ≈» 4
ALTERNATIVE 2
«334 × \(\frac{{304.5}}{{100}}\) ≈» 1017
«\(\frac{{1017}}{{253.8}}\) ≈» 4
Award [2] for correct final answer.
[2 marks]
glycerol backbone
ester formula AND linkage
Accept a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Accept condensed formula for ester.
[2 marks]
has affected consumption of trans-fats/cis-fats/saturated fats/unsaturated fats/hydrogenated/artificially altered fats
OR
reduce/eliminate trans-fats/increase in cis-fats
OR
reduce/eliminate saturated fats
OR
increase unsaturated fats
Do not accept “decrease in fat” alone.
Accept “lipid” for “fats”.
[1 mark]
«\(\frac{{29.9{\text{ g}}}}{{150.15{\text{ g mo}}{{\text{l}}^{ – 1}}}}\) =» 0.199 «mol»
«0.199 mol × 205.9 kJ mol–1 =» 41.0 «kJ»
Ignore significant figures in M1.
Award [2] for correct final answer.
Award [1 max] for incorrect significant figures in final answer.
[2 marks]
ratio of oxygen to carbon in lipids lower
OR
lipids less oxidized
OR
lipids more reduced
more energy per mass/g released when lipids are oxidized
Accept “«average» oxidation number of carbon in linoleic acid is lower” for M1.
[2 marks]

