Question
Biological pigments are coloured compounds.
The following structure is the β-carotene:
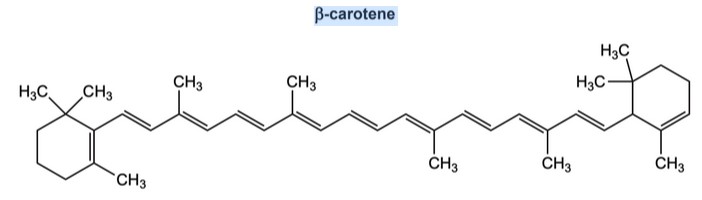
(a) Explain in terms of its structure, why β-carotene appears orange in visible white light. Refer to section 17 of the data booklet.
Anthocyanins can act as acid-base indicators. The two examples shown, are the flavylium cation and the quinoidal base.
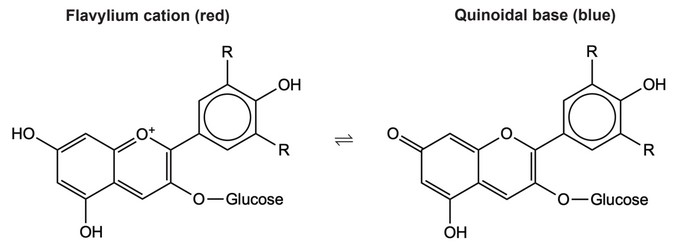
(b) Explain how these anthocyanins can act as acid-base indicators as pH increases.
Answer/Explanation
Answer:
(a) contains many/multiple conjugated «carbon–carbon/C=C» double bonds
OR
extended system of delocalized electrons
absorbs blue
OR
complementary to orange «light»
(b) Any two of:
equilibrium shifts right
OR
\(H^+\) ions lost
red to blue
loss of proton/\(H^+\) changes extent of conjugation
affects frequency/wavelength of absorbed light
complementary light transmitted
Question
DNA is an essential biological molecule.
(a) State the three components of a monomer of DNA (a nucleotide).
(b) Draw the hydrogen bonds between adenine and thymine.
Answer/Explanation
Answer:
(a) phosphate AND deoxyribose AND nitrogenous base
(b) 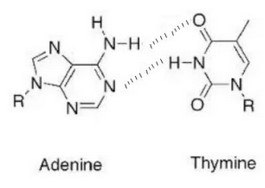
Question
a. Describe the function of chlorophyll in photosynthesis.
b. Compare and contrast the structures of starch and cellulose.
One similarity:
One difference:
c. Explain why maltose, $\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}$, is soluble in water.
▶️Answer/Explanation
Markscheme
a. absorbs/traps light «energy»
initiates redox reactions
OR
transfers electrons
b. One similarity:
1-4/glycosidic linkage
OR
glucose monomers/residues
NOTE: Accept “both are polysaccharides”.
One difference:
starch has $\alpha$-glucose $A N D$ cellulose has $\beta$-glucose «monomers»
OR
starch can form coiled/spiral/helical chains «and straight chains» $A N D$ cellulose cannot/can only form straight chains/can only form a linear structure
OR
starch «in amylopectin» also has 1-6 glycosidic links AND cellulose does not
NOTE: Accept “cellulose has alternate glucose monomers upside down with respect to each other AND starch does not”.
c. «solubility depends on forming many» $\mathrm{H}$-bonds with water
maltose has many hydroxyl/OH/oxygen atom/O «and forms many $\mathrm{H}$-bonds»
NOTE: Reference to “with water” required.
Accept “hydroxy” for “hydroxyl” but not “hydroxide/OH”.
Reference to many/several $\mathrm{OH}$ groups/O atoms required for $M 2$.
