Question
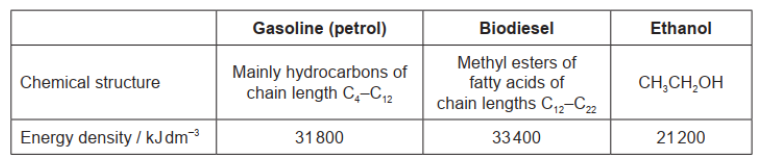
Gasoline (petrol), biodiesel and ethanol are fuels.
b. State a class of organic compounds found in gasoline.
c. Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.

d. A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
e. Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
f(i).When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
$\mathrm{f}(\mathrm{ii)}$ Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
f(iii)Suggest a wavenumber absorbed by methane gas.
▶️Answer/Explanation
Markscheme
a. $« 21200 \mathrm{~kJ} \mathrm{dm}^{-3} \times 5.00 \mathrm{dm}^3=» 106000 / 1.06 \times 10^5\langle\mathrm{~kJ}\rangle$
b. alkane
OR
cycloalkane
OR
arene
Accept “alkene”.
Do not accept just “hydrocarbon”, since given in stem.
Do not accept “benzene/aromatic” for “arene”.
c. Advantages: [2 max]
renewable
uses up waste «such as used cooking oil»
lower carbon footprint/carbon neutral
higher flashpoint
produces less $\mathrm{SO}_{\mathrm{x}} / \mathrm{SO}_2$
OR
less polluting emissions
has lubricating properties
OR
preserves/increases lifespan of engine
increases the life of the catalytic converter
eliminates dependence on foreign suppliers
does not require pipelines/infrastructure «to produce»
relatively less destruction of habitat compared to obtaining petrochemicals
Accept “higher energy density” OR “biodegradable” for advantage.
Disadvantages: [2 max]
needs conversion/transesterification
takes time to produce/grow plants
takes up land
OR
deforestation
fertilizers/pesticides/phosphates/nitrates «used in production of crops» have negative environmental effects
biodiversity affected
OR
loss of habitats «due to energy crop plantations»
cannot be used at low temperatures
variable quality «in production»
high viscosity/can clog/damage engines
Accept “lower specific energy” as disadvantage.
Do not accept “lower octane number” as disadvantage”.
d. Any one:
uses up fossil fuels more slowly
lower carbon footprint/CO2 emissions
undergoes more complete combustion
produces fewer particulates
higher octane number/rating
OR
less knocking
prevents fuel injection system build up
OR
helps keep engine clean
Accept an example of a suitable advantage even if repeated from 9c.
e. Any two:
biodiesel has smaller molecules/single «hydrocarbon» chain AND oil has larger molecules/multiple «hydrocarbon» chains
biodiesel is methyl/ethyl ester $\boldsymbol{A N D}$ oil has «backbone of» glycerol joined to fatty acids
biodiesel contains one ester group AND oil contains three ester groups
Do not accept properties such as “less viscous” or “lower ignition point”.
f(i).carbon dioxide allows sunlight/short wavelength radiation to pass through $\boldsymbol{A N D}$ particulates reflect/scatter/absorb sunlight
Accept “particulates reflect/scatter/absorb sunlight AND carbon dioxide does not”.
Accept ” $\mathrm{CO}_2$ absorbs IR “radiation» AND particulates reflect/scatter/absorb sunlight”.
Do not accept “traps” for “absorbs”.
$\mathrm{f}(\mathrm{ii})$ carbon dioxide is highly/more abundant «in the atmosphere»
methane is more effective/potent «as a greenhouse gas»
OR
methane/better/more effective at absorbing IR «radiation»
OR
methane has greater greenhouse factor
$O R$
methane has greater global warming potential/GWP $\boldsymbol{V}$
Accept “carbon dioxide contributes more to global warming” for M1.
f(iii)any value or range within $2850-3090 \ll \mathrm{cm}^{-1}$ »
Question
Consider the following data for butane and pentane at STP.

a. Discuss the data.
b. Outline what is meant by the degradation of energy.
▶️Answer/Explanation
Markscheme
a. «similar specific energy and» pentane has «much» larger energy density
Any two for [2 max]:
similar number of bonds/« $\mathrm{C}$ and $\mathrm{H}$ » atoms in $1 \mathrm{~kg}$ «leading to similar specific energy»
OR
only one carbon difference in structure «leading to similar specific energy»
NOTE: Accept “both are alkanes” for M2.
pentane is a liquid $A N D$ butane is a gas “at STP»
NOTE: Accept “pentane would be easier to transport”.
$1 \mathrm{~m}^3$ of pentane contains greater amount/mass than $1 \mathrm{~m}^3$ of butane
NOTE: Accept “same volume” for ” $1 \mathrm{~m}^{3 “}$ ” and “more moles” for “greater amount” for M4.
b. energy converted to heat
OR energy converted to less useful/dispersed forms
OR energy converted to forms that have lower potential to do work
OR
heat transferred to the surroundings
NOTE: Reference to energy conversion/transfer required. Do not accept reference to loss of energy.
Question
The regular rise and fall of sea levels, known as tides, can be used to generate energy.
State one advantage, other than limiting greenhouse gas emissions, and one disadvantage of tidal power.
Advantage:
Disadvantage:
▶️Answer/Explanation
Markscheme
Advantage
Any one of:
renewable $[\boldsymbol{\sim}$ ]
predictable supply [ $\boldsymbol{\sim}]$
tidal barrage may prevent flooding $[\boldsymbol{V}]$
effective at low speeds $[\boldsymbol{\sim}]$
long life-span $[\boldsymbol{\sim}]$
low cost to run $[\boldsymbol{N}]$
Disadvantage
Any one of:
cost of construction $[\boldsymbol{\sim}]$
changes/unknown effects on marine life $[\boldsymbol{V}]$
changes circulation of tides in the area $[\boldsymbol{\sim}]$
power output is variable $[\boldsymbol{V}]$
limited locations where feasible $[\boldsymbol{V}]$
equipment maintenance can be challenging $[\boldsymbol{V}]$
difficult to store energy $[\boldsymbol{V}]$
Note: Do not accept vague generalizations.
Do not accept economic issues for both advantage and disadvantage.
Do not accept sustainable.
Accept “energy” or “electricity” for “power”.
Question
This question is about fuel for engines.
a. Crude oil can be converted into fuels by fractional distillation and cracking.
Contrast these two processes.
b. Determine the specific energy, in $\mathrm{kJ} \mathrm{g}^{-1}$, and energy density, in $\mathrm{kJ} \mathrm{cm}^{-3}$, of hexane, $\mathrm{C}_6 \mathrm{H}_{14}$. Give both answers to three significant figures.
Hexane: $M_r=86.2 ; \Delta \mathrm{H}_{\mathrm{c}}=-4163 \mathrm{~kJ} \mathrm{~mol}^{-1}$; density $=0.660 \mathrm{~g} \mathrm{~cm}^{-3}$
Specific energy:
Energy density:
c. Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
▶️Answer/Explanation
Markscheme
a.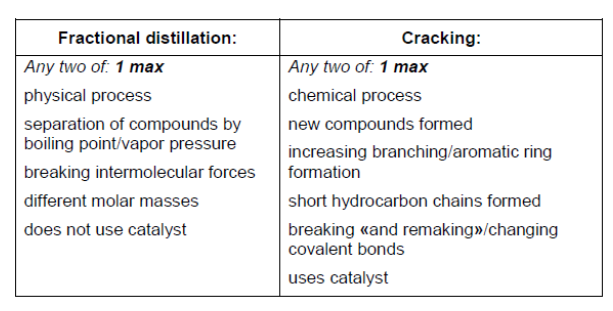
Note: Award [1] max for any two correct answers from one column OR one from each column.
Award [2] for any two correct from each column; eg: fractional distillation – any two correct award [1 max] AND cracking – any two correct award [1 $\max ]$.
b. specific energy $=« \frac{4163 \mathrm{~kJ} \mathrm{~mol}^{-1}}{86.2 \mathrm{~g} \mathrm{~mol}^{-1}}=» 48.3$ «kJ g-1»[ $\left.\mathrm{V}\right]$
energy density $=« 48.3 \mathrm{~kJ} \mathrm{~g}^{-1} \times 0.660 \mathrm{~g} \mathrm{~cm}^{-3}=» 31.9 \ll \mathrm{kJ} \mathrm{cm}^{-3} »[\mathcal{W}]$
Note: Award [1 max] if either or both answers not expressed to three significant figures.
c. Any two of:
«hydrocarbons are heated with» catalyst $[\mathfrak{V}]$
long chains break and reform
OR
branching/aromatization occurs
OR
isomerisation/reforming/platforming/cracking [ $\boldsymbol{V}$ ]
zeolite separates branched from non-branched
OR
products are distilled
$O R$
«distillation» separates reformed and cracked products [ $\boldsymbol{V}$ ]
Note: Accept a specific catalysis name or formula for M1 such as Pt/Re/Rh/Pd/Ir.
Question
Natural gas is an energy source composed mainly of methane.
Natural gas is burned to produce steam which turns turbines in an electricity generating power plant.
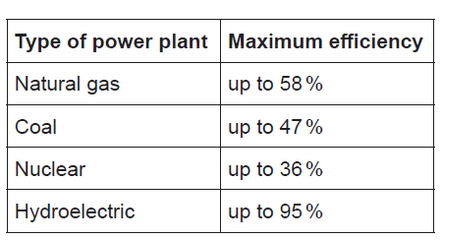
The efficiency of several sources for power plants is given below.
a. Calculate the specific energy of methane, in $\mathrm{MJ} \mathrm{kg}^{-1}$, using sections 1, 6 and 13 of the data booklet.
b(i)Calculate the maximum electric energy output, in MJ, which can be obtained from burning $1.00 \mathrm{~kg}$ of methane by using your answer from (a).
b(ii)Hydroelectric power plants produced $16 \%$ of the world’s energy in 2015, down from $21 \%$ in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
c(i)Methane can also be obtained by fractional distillation of crude oil.
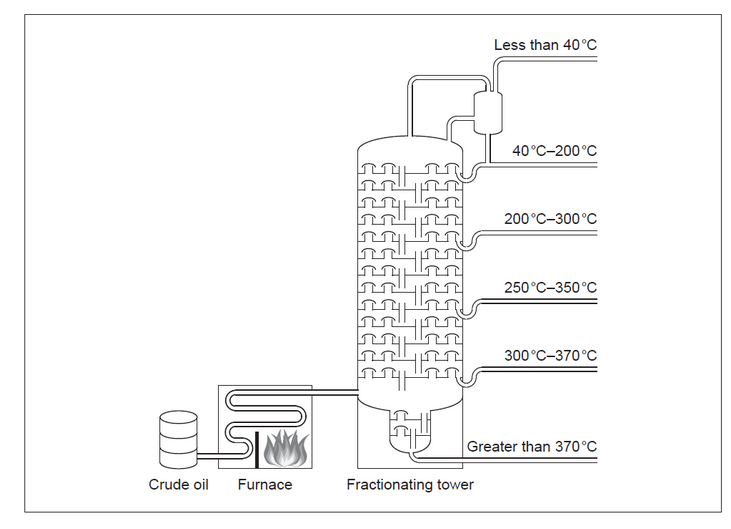
Draw a circle on the diagram to show where the methane fraction is withdrawn.
c(ii)_ist the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
d(i)Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
d(ii)Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
▶️Answer/Explanation
Markscheme
a. $« \frac{891 \mathrm{kJmol}^{-1}}{16.05 \mathrm{gmol}^{-1}}=55.5 \mathrm{~kJ} \mathrm{~g}^{-1}=» 55.5 \ll \mathrm{MJ} \mathrm{kg}^{-1}$ [ $\left.\sim\right]$
$\mathrm{b}(\mathrm{i}) \ll 55.5 \mathrm{MJ} \times 58 \%=» 32.2$ «MJ»[$[\mathbf{V}]$
b(ii)Reason for higher efficiency:
no heat/energy loss in producing steam
OR
no need to convert chemical energy of the fuel into heat and then heat into mechanical energy
$O R$
direct conversion of «gravitational» potential energy to mechanical energy
Note: Accept “less energy lost as heat” but do not accept “no energy lost”.
Reason for decreased use:
limited supply of available hydroelectric sites
OR
rapid growth of electrical supply in countries with little hydroelectric potential
OR
not building «new hydroelectric» dams because of environmental concerns [ $\mathcal{V}$ ]
Note: Accept “new/alternative/solar/wind power sources “have taken over some of the demand»”.
Accept “lower output from existing stations due to limited water supplies”.
c(i).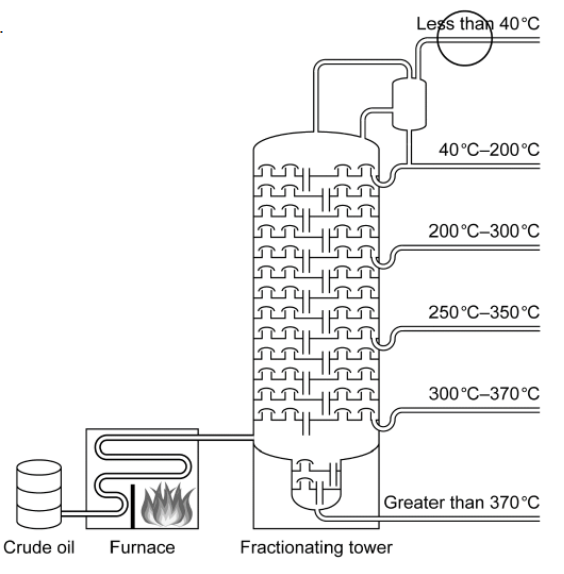
c(iigasoline $>$ diesel $>$ lubricating motor oil $>$ asphalt $[\boldsymbol{U}$ ]
Note: Accept products written in this order whether separated by $>$, comma, or nothing.
$\mathrm{d}(\mathrm{i})$ methane is tetrahedral
OR
methane has zero dipole moment/is non-polar/bond polarities cancel $[\mathcal{U}$ ]
Any two of:
IR absorption can result in increased vibrations/bending/stretching [ ]
only modes that cause change in dipole absorb IR $[\boldsymbol{\sim}]$
for methane this is asymmetric bending/stretching $[\boldsymbol{\sim}]$
d(ii)methane is less abundant $\boldsymbol{A N D}$ has a greater effect «per mol» [ $\boldsymbol{U}$ ]
Question
Hexane, C6H14, is not a suitable fuel for internal combustion engines as it has a tendency to auto-ignite, a cause of “knocking”.
(i) Hexane can be converted to different organic products in a reforming process. Identify one of these products.
(ii) Suggest why the product in (a)(i) has a lesser tendency to auto-ignite than hexane.
(i) Octane, C8H18, can undergo complete combustion under suitable conditions. Calculate the specific energy of octane, in kJg−1, using sections 1, 6 and 13 of the data booklet.
(ii) The specific energy of ethanol is 29.7kJg−1. Evaluate the addition of ethanol to octane (or its isomers) for use as a fuel in motor vehicles, giving one advantage and one disadvantage.
Advantage:
Disadvantage:
Coal can be heated with steam to produce synthetic natural gas. Formulate an equation to show the formation of methane, CH4(g), from coal, C(s), and steam, H2O(g).
▶️Answer/Explanation
Markscheme
2,2-dimethylbutane
OR
2,3-dimethylbutane
OR
3-methylpentane
OR
2-methylpentane
OR
cyclohexane
OR
methylcyclopentane
OR
benzeneAccept name or structural formula.
Accept any mono or poly-substituted cycloalkane with a total of six carbon atoms.
(ii)
increased branching (for acyclic hydrocarbons)/aromatic/aromaticity (for benzene)/cyclic hydrocarbon
OR
tertiary radicals are more stable
OR
higher octane ratingResponse in M1 must be consistent with molecule chosen in a (i)
(i)
«\(\frac{{5470}}{{114.26}}\)= » 47.9 «kJ g-1»
(ii)
Advantage:
ethanol does not produce particulates/has less incomplete combustion/CO/HCs/VOCs/is less polluting
OR
ethanol has high octane rating
OR
ethanol is renewable
OR
less environmental risks associated with spills for ethanol OR less carbon dioxide/CO2 produced if renewable energy source used
OR
economic advantages for countries that cannot produce crude oil
Accept any valid advantage and disadvantage.
Ignore any mention of cost.
Ignore any mention of NOx.
Disadvantage:
reduces efficiency/lowers specific energy/lowers energy density
OR
ethanol is more volatile/evaporates easily «than octane or its isomers»
OR
land that could be used for food production used to produce crops for ethanol
OR
biodiversity can be affected/loss of habitats «due to energy crop plantations
OR
phosphorus/nitrogen used in production has negative environmental effects
OR
modification of current engines «may be required» if ethanol used
Accept “if the fuel blend consists of nearly pure ethanol, engine is difficult to start in cold weather”.
Accept for disadvantage any engine-related problem affected by ethanol use (eg. effect on fuel pumps, incorrect fuel quantity indicators, older cars may not be suitable for ethanol use, etc.).
2C(s) + 2H2O(g) → CH4 (g) + CO2 (g)
OR
3C(s) + 2H2O(g) → CH4 (g) + 2CO(g)
Accept a two-step process.
Question
Chemical energy from redox reactions can be used as a portable source of electrical energy. A hybrid car uses a lithium ion battery in addition to gasoline as fuel.
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
▶️Answer/Explanation
Markscheme
i
«\(\frac{{1.58 \times {{10}^7}{\rm{J}}}}{{80.0{\rm{kg}}}}\)=\(\frac{{15.8{\rm{MJ}}}}{{80.0{\rm{kg}}}}\)=» 1.98 × 10−1 «MJ kg−1»
ii
gasoline releases more energy from a given mass of fuel
OR
gasoline has higher specific energy
Do not accept volume in place of mass as question refers to specific energy, not energy density.
i
«\(\frac{{15.8\,{\text{MJ}}}}{{34.3\,{\text{MJ}}\,{\text{d}}{{\text{m}}^{ – 3}}}}\)»= 4.61 × 10−1 «dm3»
ii
«4.61 × 10−1 dm3 × 32.0 km dm−3 × 4»= 59.0/59.1 «km»
Question
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
State two reagents required to convert vegetable oil to biodiesel.
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
Determine the specific energy, in kJ\(\,\)g−1, and energy density, in kJ\(\,\)cm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 g\(\,\)cm−3; Molar mass = 299 g\(\,\)mol−1;
Enthalpy of combustion = 12.0 MJ\(\,\)mol−1.
▶️Answer/Explanation
Markscheme
methanol
OR
ethanol
strong acid
OR
strong base
Accept “alcohol”.
Accept any specific strong acid or strong base other than HNO3/nitric acid.
[3 marks]
CH3(CH2)16COOCH3 / CH3OCO(CH2)16CH3
OR
CH3(CH2)16COOC2H5 / C2H5OCO(CH2)16CH3
Product must correspond to alcohol chosen in (a), but award mark for either structure if neither given for (a).
[1 mark]
lower viscosity
weaker intermolecular/dispersion/London/van der Waals’ forces
OR
smaller/shorter molecules
Accept “lower molecular mass/Mr” or “lower number of electrons”.
Accept converse arguments.
[2 marks]
Specific energy: «\( = \frac{{12\,000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}{{299{\text{ g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)» = 40.1 «kJ g−1»
Energy density: «= 40.1 kJ\(\,\)g−1 x 0.850 g\(\,\)cm−3» = 34.1 «kJ\(\,\)cm−3»
Award [1] if both are in terms of a unit other than kJ (such as J or MJ).
[2 marks]
Question
There are many sources of energy available.
State one advantage and one disadvantage for each energy source in the table.
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.
▶️Answer/Explanation
Markscheme
Do not award marks for converse statements for advantage and disadvantage.
Points related to greenhouse gases should be counted only once for the entire question.
Biofuels:
Accept “«close to» carbon neutral”, “produce less greenhouse gases/CO2” as an advantage.
Accept “engines have to be modified if biodiesel used” as a disadvantage.
Fossil Fuels:
Accept specific pollution examples (eg, oil spills, toxic substances released when burning crude oil, etc.) as a disadvantage.
[4 marks]
«specific energy =» 142
kJ\(\,\)g–1
Accept other correct values with the correct corresponding units.
M2 can be scored independently.
[2 marks]
large volumes of hydrogen required
OR
hydrogen has lower energy density
not easily transportable «form» as it is a gas
OR
heavy containers required to carry AND compress/regulate «hydrogen»
OR
high energy/cost required to compress hydrogen to transportable liquid form
OR
atmospheric pollution may be generated during production of hydrogen
OR
hydrogen fuel cells do not work at very low temperatures
OR
highly flammable when compressed/difficult to extinguish fires
OR
leaks not easy to detect
OR
high cost of production
OR
lack of filling stations/availability to consumer «in many countries»
Accept “«hydrogen combustion contributes to» knocking in engines” OR “modified engine required” for M2.
Accept “explosive” but not “more dangerous” for M2.
[2 marks]
Question
One method of comparing fuels is by considering their specific energies.
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
State the name of one renewable source of energy other than wood.
▶️Answer/Explanation
Markscheme
Mr (C8H18) = 114.26 AND ΔH\(_c^\theta \)= –5470 «kJ mol–1»
«specific energy = \(\frac{{5470\,{\text{kJ}}}}{{0.11426\,{\text{kg}}}}\) =» 4.79 x 104/47873/47900 «kJ kg–1»
Award [2] for correct final answer.
Accept “48 x 103 «kJ kg–1»” OR “47.9 x 103 «kJ kg–1»”.
wood is less useful because it requires «about three times» more mass for same energy
Accept “octane is more useful because it has higher specific energy”.
Any one of:
wind
tidal/wave
hydro-electric
solar
thermal/geothermal
plant oil
Accept “biofuel/biodiesel/«bio»ethanol” but not just “water” or “fuel cells”.
[Max 1 Mark]
Question
The increased concentration of carbon dioxide in the atmosphere is thought to result from the increased combustion of fossil fuels such as petroleum.
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
Outline why the energy available from an engine will be less than these theoretical values.
▶️Answer/Explanation
Markscheme
nitrogen/N
OR
oxygen/O
OR
sulfur/S
Accept “phosphorus/P”.
[1 mark]
Any three of:
different molar masses
OR
different strengths of intermolecular forces
different boiling points
temperature in «fractionating» column decreases upwards
«components» condense at different temperatures/heights
OR
«component with» lower boiling point leaves column first
[3 marks]
specific energy «= \(\frac{{{\text{energy released}}}}{{{\text{mass consumed}}}}\) = \(\frac{{5470{\text{ kJ mo}}{{\text{l}}^{ – 1}}}}{{114.26{\text{ g mo}}{{\text{l}}^{ – 1}}}}\)» = 47.9 «kJ g–1»
energy density «\(\frac{{{\text{energy released}}}}{{{\text{volume consumed}}}}\) = specific energy × density = 47.9 kJ g–1 × 0.703 g cm–3» = 33.7 «kJ cm–3 »
Do not accept “–47.9 «kJ g–1»”.
Do not accept “–33.7 «kJ cm–3»” unless “–47.9 «kJ g–1»” already penalized.
[2 marks]
energy is lost «to the surroundings» as heat/sound/friction
OR
energy is lost to the surroundings «as heat/sound/friction»
OR
incomplete combustion
Do not accept just “energy is lost”.
[1 mark]
Question
Crude oil is a useful energy resource.
Outline two reasons why oil is one of the world’s significant energy sources.
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
Outline how higher octane fuels help eliminate “knocking” in engines.
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
▶️Answer/Explanation
Markscheme
Any two of:
high energy content/high energy density/high specific energy
OR
high enthalpy of combustion/very exothermic enthalpy of combustion
shortage of alternatives
OR
alternatives are expensive
OR
oil is relatively cheap
OR
oil is «still» abundant/common
well-established technology
OR
easy for consumers to obtain
OR
commonly used
easy to store
OR
easy to transport
OR
easy to extract
produces energy at a reasonable rate
Accept “high potential energy” for M1.
[2 marks]
C16H34(g) → C8H16(g) + C8H18(g)
OR
C16H34(g) + H2(g) → 2 C8H18(g)
[1 mark]
C8H18 AND is an alkane
OR
C8H18 AND petrol does not contain alkenes
[1 mark]
fuels can be compressed more without undergoing «unwanted» auto-ignition
Accept “burns smoother without undergoing «unwanted» auto-ignition” OR “fuel does not auto-ignite”.
[1 mark]
produces more branched chain hydrocarbons «with higher octane rating»
OR
produces aromatics «which have higher octane rating»
OR
produces cyclohexanes «which have higher octane rating»
Accept “increase branches”.
Do not accept “produces benzene”.
Do not penalize for “benzene” if penalty applied in 2.b.iii.
Accept “produces cyclic structures”.
[1 mark]
Question
The process of converting heat to electricity is limited by its thermal (Carnot) efficiency.
\({\text{Thermal efficiency}} = \frac{{{\text{temp. of steam at source (K) }}-{\text{ temp. heat sink (K)}}}}{{{\text{temp. of steam at source (K)}}}} \times 100\)
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
▶️Answer/Explanation
Markscheme
«\(\frac{{813{\text{K}} – 296{\text{K}}}}{{813{\text{K}}}}\) × 100» = 64 «%»
[1 mark]
35% of chemical/potential energy available in coal is transformed to electricity/electrical energy
not all chemical energy from burning fuel transferred into heating water
OR
energy dispersed elsewhere/energy lost due to friction of moving parts
OR
heat loss to the surroundings
Accept “stored energy” for “potential energy”.
[2 marks]

