Question
Ethanol is a biofuel that can be mixed with gasoline.
a. Write the equation for the complete combustion of ethanol.
b. Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
c. Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
d. State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
▶️Answer/Explanation
Markscheme
a. $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{l})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
b. Any two of:
«showing strong» correlation between «atmospheric» $\mathrm{CO}_2$ concentration/greenhouse gas concentration and average «global/surface/ocean» temperature
lab evidence that greenhouse gases/CO $\mathrm{CO}_2$ absorb(s) infrared radiation «advanced» computer modelling
ice core data $\checkmark$
tree ring data
ocean sediments / coral reefs / sedimentary rocks data $\checkmark$
NOTE: Do not accept “global warming” for “average temperature”.
Do not accept “traps/reflects heat” OR “thermal energy”.
Evidence must be outlined and connected to data.
Accept references to other valid greenhouse gases other than carbon dioxide/CO 2 , such as methane/CH $\mathrm{C}_4$ or nitrous oxide/ $\mathrm{N}_2 \mathrm{O}$.
c. biofuel raw material/sugar/glucose formed by photosynthesis
OR
biofuel raw material/sugar/glucose uses up carbon dioxide during its formation
OR
biofuel from capturing gases due to decaying organic matter formed from photosynthesis
$6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2$ (g)
NOTE: Accept arguments based on material coming from plant sources consuming carbon dioxide/carbon for M1.
d. transesterification
OR
«nucleophilic» substitution $/ \mathrm{S}_{\mathrm{N}}$
Question
Octane number is a measure of the performance of engine fuel.
a. Suggest why a high-octane number fuel is preferable.
b(i)Reforming reactions are used to increase the octane number of a hydrocarbon fuel.
Suggest the structural formulas of two possible products of the reforming reaction of heptane, $\mathrm{C}_7 \mathrm{H}_{16}$.
$\mathrm{b}$ (ii)The ${ }^1 \mathrm{H}$ NMR spectrum of one of the products has four signals. The integration trace shows a ratio of the areas under the signals of $9: 3: 2: 2$.
Deduce the structural formula of the product.
▶️Answer/Explanation
Markscheme
a. low knocking/auto-ignition
NOTE: Do not accept “pre-ignition”.
OR
more efficient fuel
NOTE: Accept “less $\mathrm{CO}_2$ emissions since knocking engine uses more fuel “to produce the same power»”.
OR
high compression
OR
more power extracted
OR
more air going into engine / turbocharging
OR
less engine damage
b(i)Any two of:
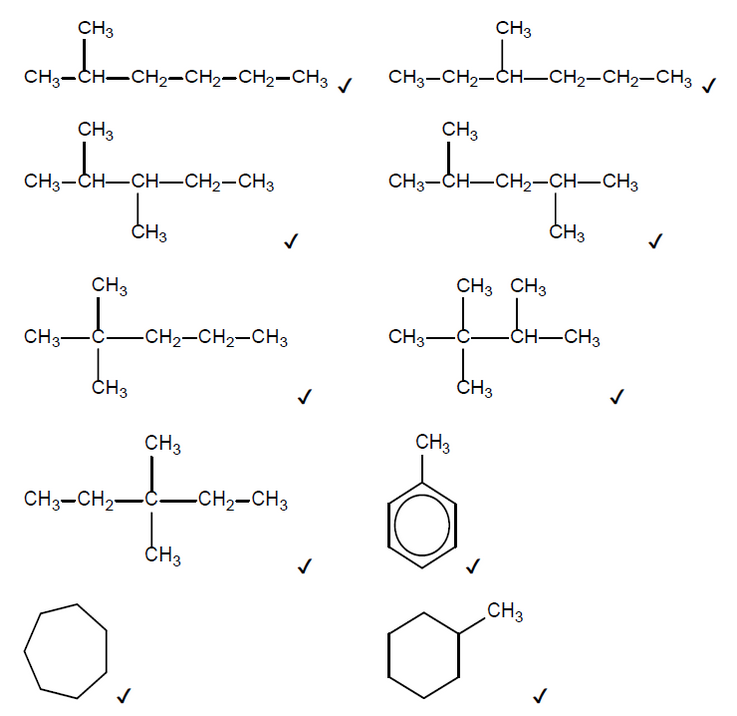
NOTE: Accept skeletal formulas or full or condensed structural formulas.
Accept any other branched cycloalkane that contains 7 carbons.
Do not accept any alkenes.
Penalise missing hydrogens or bond connectivities once only in Option C.
Accept hydrogen as the second product if the first product is toluene or a cycloalkane.
b(ii).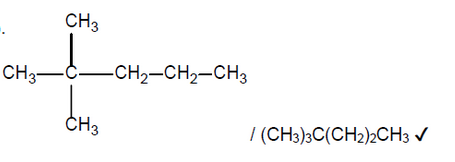
NOTE: Accept a skeletal formula or a full or condensed structural formula. Penalise missing hydrogens or bond connectivities once only in Option C.
Question
This question is about fuel for engines.
a. Crude oil can be converted into fuels by fractional distillation and cracking. Contrast these two processes.
b. Determine the specific energy, in $\mathrm{kJ} \mathrm{g}^{-1}$, and energy density, in $\mathrm{kJ} \mathrm{cm}^{-3}$, of hexane, $\mathrm{C}_6 \mathrm{H}_{14}$. Give both answers to three significant figures.
Hexane: $M_r=86.2 ; \Delta \mathrm{H}_{\mathrm{c}}=-4163 \mathrm{~kJ} \mathrm{~mol}^{-1}$; density $=0.660 \mathrm{~g} \mathrm{~cm}^{-3}$
Specific energy:
Energy density:
c. Hydrocarbons need treatment to increase their octane number to prevent pre-ignition (knocking) before they can be used in internal combustion engines.
Describe how this is carried out and the molecular changes that take place.
▶️Answer/Explanation
Markscheme
a.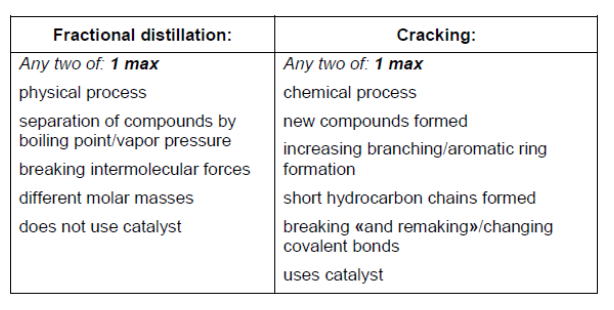
Note: Award [1] max for any two correct answers from one column OR one from each column.
Award [2] for any two correct from each column; eg: fractional distillation – any two correct award [1 max] AND cracking – any two correct award [1 $\max ]$.
b. specific energy $=« \frac{4163 \mathrm{~kJ} \mathrm{~mol}^{-1}}{86.2 \mathrm{~g} \mathrm{~mol}^{-1}}=» 48.3 « \mathrm{~kJ} \mathrm{~g}^{-1} \rrbracket[\sim]$
energy density $=« 48.3 \mathrm{~kJ} \mathrm{~g}^{-1} \times 0.660 \mathrm{~g} \mathrm{~cm}^{-3}=» 31.9 « \mathrm{~kJ} \mathrm{~cm}^{-3}$ [ $\left.\sim\right]$
Note: Award [1 max] if either or both answers not expressed to three significant figures.
c. Any two of:
«hydrocarbons are heated with» catalyst $[\boldsymbol{U}$ ]
long chains break and reform
OR
branching/aromatization occurs
OR
isomerisation/reforming/platforming/cracking [ $\sqrt{ }$ ]
zeolite separates branched from non-branched
OR
products are distilled
OR
«distillation» separates reformed and cracked products [ $\boldsymbol{U}$ ]
Note: Accept a specific catalysis name or formula for M1 such as Pt/Re/Rh/Pd/Ir.
Question
E10 is composed of $10 \%$ ethanol and $90 \%$ normal unleaded fuel.
a. Ethanol has a Research Octane Number (RON) of 108.6.
Outline how higher octane fuels affect engine performance.
b. Show that, for combustion of equal masses of fuel, ethanol $\left(M_r=46 \mathrm{~g} \mathrm{~mol}^{-1}\right)$ has a lower carbon footprint than octane $\left(M_r=114 \mathrm{~g} \mathrm{~mol}^{-1}\right)$.
c. Biodiesel containing ethanol can be made from renewable resources.
Suggest one environmental disadvantage of producing biodiesel from renewable resources.
▶️Answer/Explanation
Markscheme
a. increased $A N D$ fuels can be compressed more «before ignition» [ $\boldsymbol{U}$ ]
Note: Accept “engines can be designed with higher compression ratio” OR “less chance of pre-ignition/autoignition/ knocking occurring”.
b. Alternative 1
$$
\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{l})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}
$$
(I) / 1 mol ethanol produces $2 \mathrm{~mol} \mathrm{CO}_2$
OR
$\mathrm{C}_8 \mathrm{H} 18(\mathrm{I})+12.5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 8 \mathrm{CO}_2(\mathrm{~g})+9 \mathrm{H}_2 \mathrm{O}$
(l) $/ 1$ mol octane produces $8 \mathrm{~mol} \mathrm{CO} 2$ [ ]
For $1 \mathrm{~g}$ of fuel:
$$
\begin{aligned}
& \text { ” } \frac{1 \mathrm{~g}}{46 \mathrm{~g} \mathrm{~mol}^{-1}} \times 2 \mathrm{~mol} \mathrm{CO}_2(\mathrm{~g})=» 0.04 \ll \mathrm{mol} \mathrm{CO} \mathrm{CO}_2(\mathrm{~g}) » \text { from ethanol }[\boldsymbol{V}] \\
& \text { « } \frac{1 \mathrm{~g}}{114 \mathrm{~g} \mathrm{~mol}^{-1}} \times 8 \mathrm{~mol} \mathrm{CO}_2(\mathrm{~g})=» 0.07 « \mathrm{~mol} \mathrm{CO}_2(\mathrm{~g}) » \text { from octane [ } \\
&
\end{aligned}
$$
Alternative 2
ratio of $\mathrm{C}$ in ethanol:octane is $2: 8$, so ratio in carbon dioxide produced per mole will be $1: 4$ [ $]$ ratio amount of fuel in $1 \mathrm{~g}=\frac{1}{46}: \frac{1}{114}=2.5: 1$ [ ]
$4>2.5$ so octane produces more carbon dioxide
OR
ratio of amount of carbon dioxide $=2.5: 4=1: 1.61$ so octane produces more “for combustion of same mass” [ $\checkmark$ ]
c. use of «farm» land «for production»
$O R$
deforestation «for crop production for fuel»
$O R$
can release more $\mathrm{NO}_{\mathrm{x}}$ «than normal fuel on combustion» $[\boldsymbol{\sim}]$
Note: Ignore any reference to cost.
Question
Natural gas is an energy source composed mainly of methane.
Natural gas is burned to produce steam which turns turbines in an electricity generating power plant. The efficiency of several sources for power plants is given below.
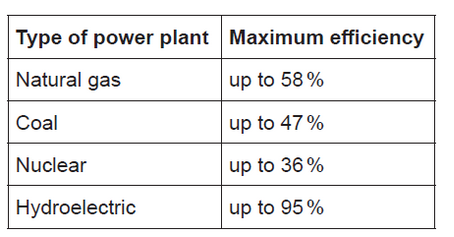
a. Calculate the specific energy of methane, in $\mathrm{MJ} \mathrm{kg}^{-1}$, using sections 1,6 and 13 of the data booklet.
$\mathrm{b}(\mathrm{i})$ Calculate the maximum electric energy output, in MJ, which can be obtained from burning $1.00 \mathrm{~kg}$ of methane by using your answer from (a). b(ii).Hydroelectric power plants produced $16 \%$ of the world’s energy in 2015, down from $21 \%$ in 1971.
Suggest why hydroelectric power production has a higher efficiency than the other sources given in (b) and why its relative use has decreased despite the high efficiency.
Reason for higher efficiency:
Reason for decreased use:
c(i)Methane can also be obtained by fractional distillation of crude oil.
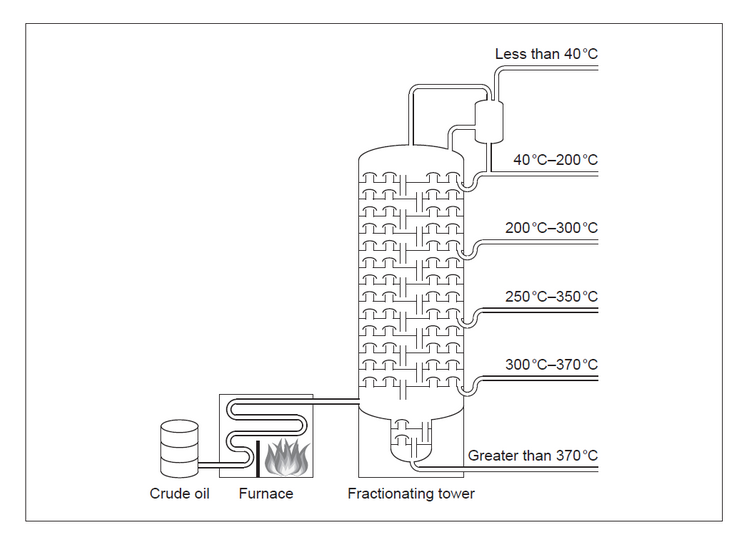
Draw a circle on the diagram to show where the methane fraction is withdrawn.
c(ii)_ist the following products, which are also obtained by fractional distillation, according to decreasing volatility: asphalt, diesel, gasoline, lubricating motor oil.
d(i)Explain how methane absorbs infrared (IR) radiation by referring to its molecular geometry and dipole moment.
d(ii)Compare methane’s atmospheric abundance and greenhouse effect to that of carbon dioxide.
▶️Answer/Explanation
Markscheme
a. $« \frac{891 \mathrm{kJmol}^{-1}}{16.05 \mathrm{gmol}^{-1}}=55.5 \mathrm{~kJ} \mathrm{~g}^{-1}=» 55.5 \ll \mathrm{MJ} \mathrm{kg}^{-1} \rrbracket[\mathcal{}]$
$\mathrm{b}(\mathrm{i}) \ll 55.5 \mathrm{MJ} \times 58 \%=» 32.2$ «MJ»[$[\mathrm{V}]$
b(ii)Reason for higher efficiency:
no heat/energy loss in producing steam
OR
no need to convert chemical energy of the fuel into heat and then heat into mechanical energy
OR
direct conversion of «gravitational» potential energy to mechanical energy $[\boldsymbol{U}]$
Note: Accept “less energy lost as heat” but do not accept “no energy lost”.
Reason for decreased use:
limited supply of available hydroelectric sites
OR
rapid growth of electrical supply in countries with little hydroelectric potential
OR
not building «new hydroelectric» dams because of environmental concerns $[\mathcal{~}]$
Note: Accept “new/alternative/solar/wind power sources “have taken over some of the demand»”.
Accept “lower output from existing stations due to limited water supplies”.
c(i).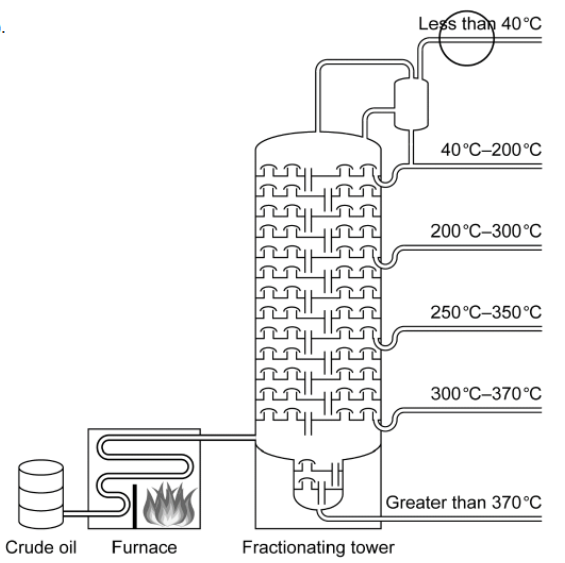
c(iigasoline $>$ diesel $>$ lubricating motor oil $>$ asphalt $[\boldsymbol{U}]$
Note: Accept products written in this order whether separated by >, comma, or nothing.
d(i)methane is tetrahedral
$O R$
methane has zero dipole moment/is non-polar/bond polarities cancel [ $\boldsymbol{U}$ ]
Any two of:
IR absorption can result in increased vibrations/bending/stretching [ $]$
only modes that cause change in dipole absorb IR $[\boldsymbol{\sim}]$
for methane this is asymmetric bending/stretching $[\boldsymbol{V}]$
d(ii)methane is less abundant $A N D$ has a greater effect «per mol» $[\mathscr{U}]$
Question
Coal can be converted to clean-burning synthetic natural gas.
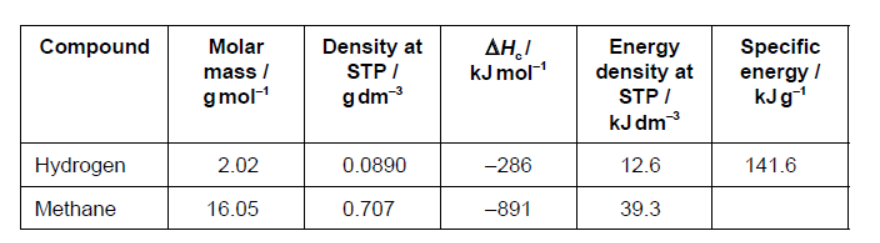
Automobile companies use hydrogen as an alternative to fossil fuels. Some properties of fuels are shown.
a. Formulate equation(s) for the conversion of coal and steam to methane.
b.i. Calculate the specific energy, in $\mathrm{kJ} \mathrm{g}^{-1}$, of methane.
b.ii.Comment on the specific energies of hydrogen and methane.
c. Calculate the mass, in $\mathrm{kg}$, of carbon dioxide produced by the complete combustion of $72.0 \mathrm{dm}^3$ octane, $\mathrm{C}_8 \mathrm{H}_{18}$.
Density of $\mathrm{C}_8 \mathrm{H}_{18}=703 \mathrm{~g} \mathrm{dm}^{-3}$
$$
\mathrm{C}_8 \mathrm{H}_{18}(\mathrm{l})+12.5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 8 \mathrm{CO}_2(\mathrm{~g})+9 \mathrm{H}_2 \mathrm{O}(\mathrm{g})
$$
▶️Answer/Explanation
Markscheme
a. ALTERNATIVE 1:
$$
2 \mathrm{C}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{CH}_4(\mathrm{~g})+\mathrm{CO}_2(\mathrm{~g})
$$
ALTERNATIVE 2:
$$
\mathrm{C}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g}) A N D 3 \mathrm{H}_2(\mathrm{~g})+\mathrm{CO}(\mathrm{g}) \rightarrow \mathrm{CH}_4(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})
$$
Accept “3C (s) $+2 \mathrm{H}_2 \mathrm{O}(g) \rightarrow \mathrm{CH}_4(g)+2 \mathrm{CO}(g)$ “.
b.i. « $\frac{891 \mathrm{~kJ} \mathrm{~mol}^{-1}}{16.05 \mathrm{~g} \mathrm{~mol}^{-1}}=» 55.5$ «kJ g ${ }^{-1}$ »
Do not penalize negative sign.
Do not accept energy density at STP/density at STP $=\frac{39.3}{0.707}=55.06$ « $\mathrm{KJ}^{-1}$ ».
b.ii.« $\frac{141.6}{55.5}$ » hydrogen/ $\mathrm{H}_2$ produces 2.6 times/more than twice the energy of methane/ $\mathrm{CH}_4$ «per mass/g»
OR
less mass of hydrogen/ $\mathrm{H}_2$ required «to produce same amount of energy»
OR
hydrogen/ $\mathrm{H}_2$ more energy efficient
c. $m_{\text {octane }} \|=72.0 \mathrm{dm}^3 \times 703 \mathrm{~g} \mathrm{dm}^{-3} »=50600 « \mathrm{~g} » / 50.6 \ll \mathrm{kg} »$
$m_{\text {carbon dioxide }} «=\frac{8 \times 44.01}{114.26} \times 50.6 »=156 « k g »$
Award [2] for correct final answer.
Question
Crude oil is a useful energy resource.
a. Outline two reasons why oil is one of the world’s significant energy sources.
b.i. Formulate an equation for the cracking of $\mathrm{C}_{16} \mathrm{H}_{34}$ into two products with eight carbon atoms each.
b.ii.dentify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
c.i. Outline how higher octane fuels help eliminate “knocking” in engines.
c.ii.The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
▶️Answer/Explanation
Markscheme
a. Any two of:
high energy content/high energy density/high specific energy
OR
high enthalpy of combustion/very exothermic enthalpy of combustion
shortage of alternatives
OR
alternatives are expensive
OR
oil is relatively cheap
OR
oil is «still» abundant/common
well-established technology
OR
easy for consumers to obtain
OR
commonly used
easy to store
$O R$
easy to transport
OR
easy to extract
produces energy at a reasonable rate
Accept “high potential energy” for M1.
[2 marks]
b.i. $\mathrm{C}_{16} \mathrm{H}_{34}(\mathrm{~g}) \rightarrow \mathrm{C}_8 \mathrm{H}_{16}(\mathrm{~g})+\mathrm{C}_8 \mathrm{H}_{18}(\mathrm{~g})$
OR
$$
\mathrm{C}_{16} \mathrm{H}_{34}(\mathrm{~g})+\mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{C}_8 \mathrm{H}_{18}(\mathrm{~g})
$$
[1 mark]
b.ii. $\mathrm{C}_8 \mathrm{H}_{18} \boldsymbol{A N D}$ is an alkane
OR
$\mathrm{C}_8 \mathrm{H}_{18}$ AND petrol does not contain alkenes
[1 mark]
c.i. fuels can be compressed more without undergoing «unwanted» auto-ignition
Accept “burns smoother without undergoing «unwanted» auto-ignition” OR “fuel does not auto-ignite”.
c.ii.produces more branched chain hydrocarbons «with higher octane rating»
OR
produces aromatics «which have higher octane rating»
OR
produces cyclohexanes «which have higher octane rating»
Accept “increase branches”.
Do not accept “produces benzene”.
Do not penalize for “benzene” if penalty applied in 2.b.iii.
Accept “produces cyclic structures”.
[1 mark]
Question
“Oil should not be used as a source of energy because it has more important uses.” Suggest two arguments that support the continued use of oil as an energy source, and two against.
▶️Answer/Explanation
Markscheme
Arguments for: [2 max]
high energy content / high enthalpy of combustion;
shortage of alternatives;
alternatives are expensive / oil relatively cheap;
well-established technology;
easy to store;
easy to transport;
produces energy at a reasonable rate;
Arguments against: [2 max]
chemical feedstock of limited supply/OWTTE;
non-renewable;
combustion causes global warming/greenhouse gases;
combustion produces acidic gases;
Apply OWTTE throughout.
Examiners report
Candidates tended to provide vague, journalist answers rather than provide specific points to score the marks, e.g. candidates would suggest ‘pollution’ as an argument against without being specific.
Question
Thermal cracking, catalytic cracking and steam cracking are all used to convert alkane molecules into smaller molecules. Identify which one of the three types of cracking is used to crack a hexane molecule, C6H14, into propane and an alkene molecule, and state the equation involved.
▶️Answer/Explanation
Markscheme
steam cracking;
\({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{14}}}} \to {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}} + {{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\);
Ignore state symbols.
Examiners report
Most candidates were able to state the equation (in quite a few cases the molecular formula of propane was not known) for the cracking process, but only about half correctly identified steam cracking.
Question
Catalytic cracking uses heterogeneous catalysts.
The initial products of the fractional distillation of oil often undergo cracking. This can be carried out in a number of ways. State the major reason for choosing each of the following techniques.
Catalytic cracking:
Thermal cracking:
Steam cracking:
Explain how these differ from homogeneous catalysts.
Identify one disadvantage of using heterogeneous catalysts.
Many of the compounds produced by cracking are used in the manufacture of addition polymers. State the essential structural feature of these compounds and explain its importance.
The polymers often have other substances added to modify their properties. One group of additives are plasticizers. State how plasticizers modify the physical properties of polyvinyl chloride and explain at the molecular level how this is achieved.
▶️Answer/Explanation
Markscheme
Catalytic cracking:
used to produce moderate length alkanes (for fuels) / lower temperature / lower energy consumption / more control of product;
Thermal cracking:
used to crack very long chain starting material;
Steam cracking:
used to produce low molar mass alkenes (for petrochemicals);
heterogeneous catalysts in a different phase to the reactants / homogeneous catalysts in the same phase as reactants;
easily poisoned / efficiency decreases over time / forms clumps / only effective on surface / require high surface area;
carbon-carbon double bond;
breaks allowing addition reaction / allows monomers/molecules to join together/polymerize;
make the polymer more flexible;
fits between/increases separation between polymer chains / allow polymer chains to slide past each other more easily / weaken intermolecular attraction;
Examiners report
Few candidates appear to have any knowledge of the different cracking techniques, though more appeared familiar with issues relating to catalysts. Quite a number of candidates were aware carbon-carbon double bonds were needed for addition polymerization, though the nature and effect of plasticizers was less well knownd.
Question
Catalysts may be homogeneous or heterogeneous.
Distinguish between homogeneous and heterogeneous catalysts.
Discuss two factors which need to be considered when selecting a catalyst for a particular chemical process.
Identify the catalyst used in the catalytic cracking of long chain hydrocarbons and state one other condition needed.
State an equation for the catalytic cracking of the straight chain hydrocarbon pentadecane, \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{32}}}}\), to produce two products with similar masses.
▶️Answer/Explanation
Markscheme
homogeneous catalysts are in the same phase/state as reactants and heterogeneous catalysts are in a different phase/state to reactants;
should produce only the desired product / selectivity;
efficiency;
should be able to work under both mild and severe conditions / should be able to work at high temperatures;
should not produce an (unwanted) environmental impact;
cost / economic viability / OWTTE;
ease of poisoning/contamination;
(catalyst a mixture of) silica/silicon dioxide/ \({\text{Si}}{{\text{O}}_2}\) and alumina/aluminium oxide/\({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) / zeolites/aluminosilicates;
high temperature / 500 °C;
\({{\text{C}}_{15}}{{\text{H}}_{32}} \to {{\text{C}}_8}{{\text{H}}_{18}} + {{\text{C}}_7}{{\text{H}}_{14}}/{{\text{C}}_{15}}{{\text{H}}_{32}} \to {{\text{C}}_8}{{\text{H}}_{16}} + {{\text{C}}_7}{{\text{H}}_{16}}\);
Examiners report
Most candidates were aware of the differences between homogeneous and heterogeneous catalysts.
[N/A]
Few candidates could name the catalyst but knew one other condition needed for catalytic cracking.
Most correctly stated an equation for the catalytic cracking of pentadecane, but some added oxygen or water, and some had too many hydrogen atoms in the products.
Question
Petroleum (mineral oil) can be used either as a fuel or a chemical feedstock.
Name two fuels that are obtained from petroleum.
Describe one environmental problem that can result from the combustion of these fuels in the internal combustion engine and identify the specific combustion product responsible.
Plastic litter is an environmental problem that results from the use of petroleum as a chemical feedstock. Identify the property of plastics that is responsible for this.
One product that is made from crude oil is the chemical feedstock that can be used to synthesize commercial liquid-crystal displays. Discuss the properties that a substance must have to make it suitable for use as a liquid-crystal display.
▶️Answer/Explanation
Markscheme
Any two for [1]
petrol/gasoline
kerosene/paraffin/aviation fuel
diesel
fuel oil/gas oil
petroleum gas/refinery gas
global warming;
carbon dioxide;
OR
air pollution;
carbon monoxide / particulates / oxides of nitrogen/NO/\({\text{N}}{{\text{O}}_{\text{2}}}\) / \({\text{VO}}{{\text{C}}_{\text{s}}}\);
Accept oxides of sulphur/SO2.
OR
acid rain;
oxides of nitrogen/NO/\({\text{N}}{{\text{O}}_{\text{2}}}\);
Accept oxides of sulphur/SO2.
slow decomposition / not biodegradeable;
chemically stable;
liquid crystal phase over a suitable range of temperatures;
rapid switching speed;
Examiners report
In part (a) a significant number of candidates named two fuels obtained from petroleum.
A significant number of candidates described the environmental problem.
The non-biodegradable property of plastics was stated correctly by many candidates.
The properties of a material that made it suitable for use as a liquid crystal display demonstrated poor understanding by many candidates.
Question
The temperature of the Earth’s surface is currently increasing. Many scientists attribute this to an increase in the levels of greenhouse gases in the atmosphere as a result of human activity.
Explain how the interaction of greenhouse gases in the atmosphere with radiation could lead to an increase in the temperature of the Earth’s surface.
Suggest why carbon dioxide is the greenhouse gas most frequently connected with the effect of human activity.
Other than carbon dioxide and water, identify one other greenhouse gas and state its source.
▶️Answer/Explanation
Markscheme
shorter wavelength/UV/high energy radiation from sun passes through;
long wavelength/infrared/IR radiation from Earth’s surface (some of this radiation) is absorbed (by gas);
Do not accept “trapped” or “blocked”.
Do not accept “IR from sun”.
causes (increased) vibration in bonds;
re-radiates heat back to the Earth;
Do not accept “reflects/bounces”.
higher concentration / more abundant/increased combustion of fossil fuels (than other anthropogenic sources);
methane/\({\text{C}}{{\text{H}}_{\text{4}}}\);
decomposition of organic matter / animals / oil fields / gas fields / intensive farming / landfills;
OR
dinitrogen monoxide/nitrous oxide/\({{\text{N}}_{\text{2}}}{\text{O}}\);
Do not accept NOx, NO, NO2, nitrogen oxides.
decomposition of organic matter/fertilizers;
OR
ozone/\({{\text{O}}_{\text{3}}}\);
photochemical smog;
OR
CFCs;
aerosol cans / air conditioners / solvents / foam production / refrigerants;
OR
sulfur hexafluoride/\({\text{S}}{{\text{F}}_{\text{6}}}\);
electrical insulator;
OR
nitrogen trifluoride/\({\text{N}}{{\text{F}}_{\text{3}}}\);
production of electronic components;
[1] for any correct gas and [1] for the corresponding source.
Examiners report
There seems to be a very poor understanding of the interaction of greenhouse gases with radiation although this question has frequently appeared in the examinations. A surprising number quoted ozone depletion and the use of terms often used in the media e.g. “trapped”, “bounces”.
In part (b), the candidates failed to state the increased combustion of fossil fuels.
Although the question stated “other than carbon dioxide and water identify one other green house gas”, many candidates identified \({\text{C}}{{\text{O}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\).
Question
the oil industry surplus long-chain hydrocarbons are converted into shorter, more useful hydrocarbons by various kinds of cracking.
State whether each of the following are examples of homogeneous or heterogeneous catalysis.
Steam cracking:
Catalytic cracking:
Hydrocracking:
▶️Answer/Explanation
Markscheme
Steam cracking:
homogeneous;
Catalytic cracking:
heterogeneous;
Hydrocracking:
heterogeneous;
Examiners report
There were few three-mark answers; perhaps many candidates guessed.
Question
Cracking is the process by which long-chain alkanes found in oil are broken down into smaller molecules.
The following reaction occurs during the cracking of tetradecane, \({{\text{C}}_{{\text{14}}}}{{\text{H}}_{{\text{30}}}}\).
\[{{\text{C}}_{14}}{{\text{H}}_{30}}{\text{(g)}} \to {{\text{C}}_{10}}{{\text{H}}_{22}}{\text{(g)}} + {\text{2}}{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}}\]
Suggest a use for each of the products formed in the reaction.
\({{\text{C}}_{{\text{10}}}}{{\text{H}}_{{\text{22}}}}\):
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\):
State the main type of product obtained from steam cracking.
Catalytic cracking uses silica as a heterogeneous catalyst. Explain the mode of action of a heterogeneous catalyst.
State one advantage of using a heterogeneous catalyst rather than a homogeneous catalyst.
Discuss two factors that need to be considered when choosing a catalyst for a process.
▶️Answer/Explanation
Markscheme
C10H22: gasoline/petrol / fuel / kerosene;
Do not allow just combustion or cars.
Allow gas for cars/automobiles instead of gasoline but not gas alone.
C2H4: chemical feedstock / OWTTE;
Accept suitable example such as manufacturing plastics/polymers but not just plastics.
alkenes;
solid surface has active sites / reactants adsorb on solid surface;
Do not accept absorb instead of adsorb.
brings reactants close together in correct orientation;
weakens reactant bonds / reactants bonds are easier to break;
can be easily removed/filtered from reaction mixture / large amount of reactant molecules pass over catalyst that is in a fixed position / can be used at high temperatures;
selectivity to produce (a high yield of) the desired product / OWTTE;
extent to which rate of reaction is increased/Ea is lowered;
amount of reactant converted to product per amount of catalyst;
Accept efficiency / conversion rate.
ability to work under different/a range of conditions;
environmental/health impact;
catalytic poisoning / active sites become blocked;
cost in relation to life expectancy / OWTTE;
ease of removal from reaction mixture;
Examiners report
Less than half of the candidates knew the uses of the products of cracking in (a) and very few candidates knew the product of steam cracking in (b).
Less than half of the candidates knew the uses of the products of cracking in (a) and very few candidates knew the product of steam cracking in (b).
The mode of action of heterogeneous catalysts was also not well answered. The majority of candidates wrote about catalysts in general gaining no marks on part (c).
Parts (d) and (e) about the advantage of heterogeneous catalysts over homogeneous catalysts, and factors to be considered when selecting a catalyst were well answered by the majority of candidates.
Parts (d) and (e) about the advantage of heterogeneous catalysts over homogeneous catalysts, and factors to be considered when selecting a catalyst were well answered by the majority of candidates.
Question
Although crude oil is considered an extremely important energy source, it cannot be used directly as a resource.
Suggest why crude oil needs to be refined before it can be used.
Thermal cracking, catalytic cracking and steam cracking can all be used to convert molecules of alkanes into alkenes.
(i) State the type of cracking which can be used to crack ethane into ethene, the chemical equation for the process and one reaction condition required.
Type of cracking:
Chemical equation:
Reaction condition:
(ii) Suggest one use for the other product formed in this reaction in addition to ethene.
▶️Answer/Explanation
Markscheme
viscous / varied composition / complex mixture / has to be broken down into more usable substances / OWTTE;
(i) Type of cracking:
steam;
Chemical equation:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {{\text{H}}_{\text{2}}}\);
One reaction condition:
high temperature range / low pressure;
Allow any temperature if specified in the range 800–1400 °C/1073–1673 K.
Award [2] for all three correct, [1] for any two correct.
(ii) fuel (eg, in space vehicles) / to make fertilizer (on reaction with nitrogen) / margarine manufacture / reduction of metal ores;
Do not apply ECF from (i).
Accept other reasonable answers.
Examiners report
Candidates struggled with this option. The few who attempted this option had difficulties with almost all the questions. For part (a) ‘refined’ was interpreted as impurities that needed to be removed. The idea of different fractions used as fuels in the crude oil was missed by majority of the candidates. Many did not provide a complete response by comparing crude oil as a fuel and as a feedstock; many only addressed one of these two. Candidates also had difficulty providing examples for crude oil components and feedstock. Students did not score well in this part. Part (b) was also challenging for the students. Most students performed poorly unable to provide examples for feedstock and crude oil fuel fractions. About half the candidates gave thermal cracking as the response for (c)(i), and were not able to score the point. Majority of the candidates gave the correct response for the uses of the products of cracking for (c)(ii).
Question
There has been a shift in the use of crude oil (petroleum) away from its use as an energy source and towards its use as a chemical feedstock.
Suggest two reasons for this shift.
A lot of feedstock is used in the production of plastics. Discuss two advantages and one disadvantage of using plastic for packaging instead of cardboard.
Two advantages:
One disadvantage:
▶️Answer/Explanation
Markscheme
increasing cost of oil (relative to other energy sources);
limited supply (of petroleum);
other sources of energy available / alternative energy sources;
(use as a raw material) reduces/delays greenhouse gas/global warming/climate change problems;
concerns about greenhouse gases/climate change causing changes in behaviour / OWTTE;
Do not accept just “greenhouse gases/climate change”;
products from raw materials can be recycled / fuels cannot be recycled;
increasing demand as raw material from continued economic growth/demand for wider variety of products;
more profit to be made (by using as raw material);
reduced availability of other sources of hydrocarbons;
Accept political factors, such as “conflicts disrupting production”.
Advantages:
Any two for [2 max] of:
waterproof so strong when wet;
can be transparent so contents can be seen;
better insulates the item it is packing if expanded plastic/bubble wrap used;
can be vacuum sealed to exclude air/keep food fresh;
better protection against knocks as it can be moulded to fit the item;
Disadvantages:
Any one of:
uses valuable petroleum resources which are non-renewable;
(may) not be burned safely because toxic gases are produced;
(may) not be bio-degradable/recyclable so will linger in landfill;
Accept other valid answers for both advantages and disadvantages.
Each answer must be qualified.
Examiners report
Many candidates did achieve at least one mark, usually referring to the increasing demand of crude oil as a raw material linked to demand for wider variety of products. Any other reasons were often inadequately communicated. There were many responses referring to the ‘production of greenhouse gases’ with no further qualification with respect to the shift in behaviour. The second part of this question produced answers which often failed to precisely address the advantages and disadvantages of the use of plastics versus cardboard specifically for packaging.
Question
Hexane, C6H14, is not a suitable fuel for internal combustion engines as it has a tendency to auto-ignite, a cause of “knocking”.
(i) Hexane can be converted to different organic products in a reforming process. Identify one of these products.
(ii) Suggest why the product in (a)(i) has a lesser tendency to auto-ignite than hexane.
(i) Octane, C8H18, can undergo complete combustion under suitable conditions. Calculate the specific energy of octane, in kJg−1, using sections 1, 6 and 13 of the data booklet.
(ii) The specific energy of ethanol is 29.7kJg−1. Evaluate the addition of ethanol to octane (or its isomers) for use as a fuel in motor vehicles, giving one advantage and one disadvantage.
Advantage:
Disadvantage
Coal can be heated with steam to produce synthetic natural gas. Formulate an equation to show the formation of methane, CH4(g), from coal, C(s), and steam, H2O(g).
▶️Answer/Explanation
Markscheme
2,2-dimethylbutane
OR
2,3-dimethylbutane
OR
3-methylpentane
OR
2-methylpentane
OR
cyclohexane
OR
methylcyclopentane
OR
benzeneAccept name or structural formula.
Accept any mono or poly-substituted cycloalkane with a total of six carbon atoms.
(ii)
increased branching (for acyclic hydrocarbons)/aromatic/aromaticity (for benzene)/cyclic hydrocarbon
OR
tertiary radicals are more stable
OR
higher octane ratingResponse in M1 must be consistent with molecule chosen in a (i)
(i)
«\(\frac{{5470}}{{114.26}}\)= » 47.9 «kJ g-1»
(ii)
Advantage:
ethanol does not produce particulates/has less incomplete combustion/CO/HCs/VOCs/is less polluting
OR
ethanol has high octane rating
OR
ethanol is renewable
OR
less environmental risks associated with spills for ethanol OR less carbon dioxide/CO2 produced if renewable energy source used
OR
economic advantages for countries that cannot produce crude oil
Accept any valid advantage and disadvantage.
Ignore any mention of cost.
Ignore any mention of NOx.
Disadvantage:
reduces efficiency/lowers specific energy/lowers energy density
OR
ethanol is more volatile/evaporates easily «than octane or its isomers»
OR
land that could be used for food production used to produce crops for ethanol
OR
biodiversity can be affected/loss of habitats «due to energy crop plantations
OR
phosphorus/nitrogen used in production has negative environmental effects
OR
modification of current engines «may be required» if ethanol used
Accept “if the fuel blend consists of nearly pure ethanol, engine is difficult to start in cold weather”.
Accept for disadvantage any engine-related problem affected by ethanol use (eg. effect on fuel pumps, incorrect fuel quantity indicators, older cars may not be suitable for ethanol use, etc.).
2C(s) + 2H2O(g) → CH4 (g) + CO2 (g)
OR
3C(s) + 2H2O(g) → CH4 (g) + 2CO(g)
Accept a two-step process.
Question
Auto-ignition of hydrocarbon fuel in a car engine causes “knocking”. The tendency of a fuel to knock depends on its molecular structure.
Discuss how the octane number changes with the molecular structure of the alkanes.
Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the equation for the conversion of heptane to methylbenzene.
▶️Answer/Explanation
Markscheme
«tends to» decrease with longer/larger/heavier alkanes
«tends to» increase with bulkier/more branched alkanes
Accept “octane number decreases with the separation between branches” OR “increases with the more central position of branches”.
Accept converse arguments.
C7H16 → C6H5CH3 + 4H2
Accept “C7H8” for “C6H5CH3”.
Question
Coal is often converted to liquid hydrocarbon fuels through initial conversion to carbon monoxide and hydrogen.
State how these gases are produced, giving the appropriate equation(s).
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
▶️Answer/Explanation
Markscheme
heat/react with «oxygen and» water/steam
C + H2O → CO + H2
OR
3C + O2 + H2O → H2 + 3CO
OR
2C + O2 → 2CO AND C + H2O → H2 + CO
OR
C + O2 → CO2 AND C + CO2 → 2CO AND C + H2O → H2 + CO
M1 requires concept of heat.
[2 marks]
«Fischer-Tropsch» catalytic reduction of carbon monoxide with hydrogen
OR
(2n + 1)H2 + n\(\,\)CO → CnH(2n + 2) + n\(\,\)H2O
OR
reduction of carbon monoxide to methanol AND catalytic dehydration
OR
2H2 + CO + CH3OH AND n\(\,\)CH3OH → CnH2n + n\(\,\)H2O
If equation is given for a specific alkane or alkene, it must be a liquid (n > 4).
[1 mark]
Question
The combustion of fossil fuels produces large amounts of CO2, a greenhouse gas.
The diagram below illustrates a range of wavelengths in the electromagnetic spectrum.
Synthesis gas, or syngas, mainly composed of CO(g) and H2(g), is an alternative form of fuel. It can be produced by coal or biomass gasification, passing steam over the source material in a low oxygen environment.
Identify which region, A or B, corresponds to each type of radiation by completing the table.
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) \( \rightleftharpoons \) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
Suggest an equation for the production of syngas from coal.
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
Suggest a reason why syngas may be considered a viable alternative to crude oil.
▶️Answer/Explanation
Markscheme
Accept “B” alone for incoming radiation from sun.
All three correct answers necessary for mark.
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)
State symbols AND equilibrium arrow required for mark.
Accept
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq).
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq).
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
OR
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + H2O(l) \( \rightleftharpoons \) H3O+(aq) + HCO3–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + 2H2O(l) \( \rightleftharpoons \) 2H3O+(aq) + CO32–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
equilibrium shifts to the right causing increase in [H3O+]/[H+ ] «thereby decreasing pH»
Equilibrium sign needed in (b) (ii) but penalize missing equilibrium sign once only in b (i) and (ii).
Do not accept “CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)” unless equation was not given in b (i).
[2 marks]
C(s) + H2O(g) → CO(g) + H2(g)
OR
3C(s) + H2O(g) + O2(g) → 3CO(g) + H2(g)
OR
4C(s) + 2H2O(g) + O2(g) → 4CO(g) + 2H2(g)
OR
5C(s) + H2O(g) + 2O2(g) → 5CO(g) + H2(g)
Accept other correctly balanced equations which produce both CO AND H2.
[1 mark]
8CO(g) + 17H2(g) → C8H18(l) + 8H2O(g)
[1 mark]
coal more plentiful than crude oil
OR
syngas can be produced from biomass/renewable source
OR
syngas can undergo liquefaction to form octanes/no need to transport crude
OR
syngas can be produced by gasification underground, using carbon
OR
capture/storage «to not release CO2 to the atmosphere»
OR
coal gasification produces other usable products/slag
[1 mark]
Question
Much of our energy needs are still provided by the refined products of crude oil.
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
Outline how water and carbon dioxide absorb infrared radiation.
▶️Answer/Explanation
Markscheme
CH3CH2CH2CH2CH2CH2CH3 → CH3CH(CH3)CH2CH(CH3)2
OR
CH3CH2CH2CH2CH2CH2CH3 →
isomerisation/reforming/platforming/cracking
Pt/Re/Rh/Pd/Ir
OR
catalyst
A structural formula is only required for the organic product, not heptane.
Accept any correctly balanced equation showing increased branching or cyclization OR aromatization OR cracking.
Suitable supports for catalysts may be included for M3 (eg silica, alumina, zeolite) but the symbol or name of an appropriate metal must be given (typically a noble metal). Ignore temperature and other conditions.
Award M2 AND M3 for “catalytic isomerisation” OR “catalytic reforming” OR “catalytic cracking”.
which specific carbon-based greenhouse gases are included
OR
whether non-carbon based greenhouse gases should be included
OR
whether CO/incomplete combustion should be included «as can be oxidized to CO2»
OR
how to “sum” all steps in a process creating CO2
OR
difficult to determine both direct and indirect production of GHG/greenhouse gas emissions
Ignore reference to geopolitical issues (eg false recording of data by governments etc.).
Accept “difficult to measure all sources of CO2” but not “difficult to measure CO2 released in atmosphere”.
Any three of:
incoming solar radiation is short wavelength/high frequency/high energy/UV
radiated/emitted as long wavelength/low frequency/low energy/IR «radiation»
energy/IR «radiation» absorbed by «bonds in» greenhouse gases
energy radiated/emitted as IR «radiation» some of which returns back to Earth
Do not accept “reflected” OR “bounced” OR “trapped”.
[Max 3 Marks]
bond length changes
OR
«asymmetric» stretching «of bonds»
OR
bond angle changes/bends
OR
polarity/dipole «moment» changes
OR
a dipole «moment» is created «when the molecule absorbs IR»
Accept “vibration of bonds” OR appropriate diagram
Question
The increased concentration of carbon dioxide in the atmosphere is thought to result from the increased combustion of fossil fuels such as petroleum.
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
Outline why the energy available from an engine will be less than these theoretical values.
▶️Answer/Explanation
Markscheme
nitrogen/N
OR
oxygen/O
OR
sulfur/S
Accept “phosphorus/P”.
[1 mark]
Any three of:
different molar masses
OR
different strengths of intermolecular forces
different boiling points
temperature in «fractionating» column decreases upwards
«components» condense at different temperatures/heights
OR
«component with» lower boiling point leaves column first
[3 marks]
specific energy «= \(\frac{{{\text{energy released}}}}{{{\text{mass consumed}}}}\) = \(\frac{{5470{\text{ kJ mo}}{{\text{l}}^{ – 1}}}}{{114.26{\text{ g mo}}{{\text{l}}^{ – 1}}}}\)» = 47.9 «kJ g–1»
energy density «\(\frac{{{\text{energy released}}}}{{{\text{volume consumed}}}}\) = specific energy × density = 47.9 kJ g–1 × 0.703 g cm–3» = 33.7 «kJ cm–3 »
Do not accept “–47.9 «kJ g–1»”.
Do not accept “–33.7 «kJ cm–3»” unless “–47.9 «kJ g–1»” already penalized.
[2 marks]
energy is lost «to the surroundings» as heat/sound/friction
OR
energy is lost to the surroundings «as heat/sound/friction»
OR
incomplete combustion
Do not accept just “energy is lost”.
[1 mark]
Question
Crude oil is a useful energy resource.
Outline two reasons why oil is one of the world’s significant energy sources.
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
Outline how higher octane fuels help eliminate “knocking” in engines.
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
▶️Answer/Explanation
Markscheme
Any two of:
high energy content/high energy density/high specific energy
OR
high enthalpy of combustion/very exothermic enthalpy of combustion
shortage of alternatives
OR
alternatives are expensive
OR
oil is relatively cheap
OR
oil is «still» abundant/common
well-established technology
OR
easy for consumers to obtain
OR
commonly used
easy to store
OR
easy to transport
OR
easy to extract
produces energy at a reasonable rate
Accept “high potential energy” for M1.
[2 marks]
C16H34(g) → C8H16(g) + C8H18(g)
OR
C16H34(g) + H2(g) → 2 C8H18(g)
[1 mark]
C8H18 AND is an alkane
OR
C8H18 AND petrol does not contain alkenes
[1 mark]
fuels can be compressed more without undergoing «unwanted» auto-ignition
Accept “burns smoother without undergoing «unwanted» auto-ignition” OR “fuel does not auto-ignite”.
[1 mark]
produces more branched chain hydrocarbons «with higher octane rating»
OR
produces aromatics «which have higher octane rating»
OR
produces cyclohexanes «which have higher octane rating»
Accept “increase branches”.
Do not accept “produces benzene”.
Do not penalize for “benzene” if penalty applied in 2.b.iii.
Accept “produces cyclic structures”.
[1 mark]

