Question
Aspirin is most commonly used as a mild analgesic. State two other common medical uses for aspirin.
Answer/Explanation
Answer:
Any two of:
reduce fever/antipyretic
anti-inflammatory
anti-coagulant/reduces blood clotting/blood thinner
OR
prevent cardiovascular disease/stroke
Question
Suggest two reasons why the penicillin side-chain is modified.
Answer/Explanation
Answer:
bacterial resistance «to older penicillin’s/antibiotics»
prevent penicillinase/beta-lactamase/enzyme in bacterium to deactivate/open
penicillin/beta-lactam ring
Question
Aspirin is formed by reacting salicylic acid with ethanoic anhydride. The structure of aspirin is given in section 37 of the data booklet.
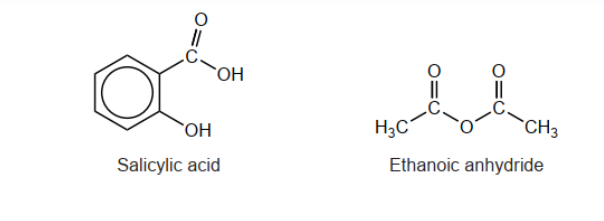
a. Deduce the structural formula of the by-product of this reaction.
b. Aspirin crystals are rinsed with water after recrystallization to remove impurities.
Suggest why cold water is used.
c. The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
d. Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing $70 \mathrm{~kg}$ :
Minimum therapeutic dose $=0.5 \mathrm{~g}$
Estimated minimum lethal dose $=15 \mathrm{~g}$
e. Explain how IR spectroscopy can be used to distinguish aspirin from salicylic acid.
▶️Answer/Explanation
Markscheme
a.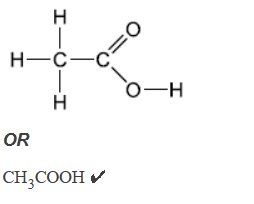
Accept full OR condensed structural formula.
b. to avoid dissolving the crystals/aspirin
Accept “to avoid loss of product” OR “aspirin is less soluble in cold water”.
c.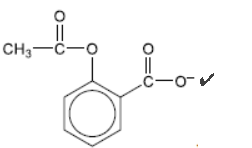
Accept a positive metal ion next to the $\mathrm{COO}^{-}$such as ” $\mathrm{Na}^{-} / \mathrm{K}^{+}$”.
Accept “-ONal – OK” without showing the charges.
Accept notations such as “RCOO” OR “RCOONa” OR “RCOOK” but not “RO-” OR “RONa” OR “ROK”.
d. low/medium risk «of overdosing» $A N D$ «estimated» lethal dose is 30 times/much larger than therapeutic dose $O R$ 30 times the dose results in chance of dying
Accept “30 and low/medium risk due to large therapeutic index”.
Do not accept “low/medium risk AND large therapeutic window”.
Do not accept “30 times the dose” alone for the mark.
e. salicylic acid contains absorption in the range $3200-3600$ «cm $^{-1} »$ due to phenol/hydroxyl/OH group not present in aspirin
Award [2] for “additional $\mathrm{OH}$ “stretch» in IR for salicylic acid at higher wavenumber than corresponding $\mathrm{OH}$ “stretch» in aspirin” $\mathrm{OR}$ “aspirin has two absorption bands/one stronger absorption band in 1700-1750 “c $\mathrm{cm}^{-1}$ “while salicylic acid has one/weaker absorption band in that region”.
Award [1 max] for “fingerprint regions will be different for both”.
