Question
Opium and its derivatives have been used for thousands of years as strong analgesics.
a. Explain how opiates act to provide pain relief.
b. Discuss how the difference in structure of two opiates, codeine and morphine, affect their ability to cross the blood-brain barrier. Use section 37 of the data booklet.
▶️Answer/Explanation
Markscheme
a. «temporarily» bond/bind to «opioid» receptors in the brain/CNS $[\boldsymbol{U}]$
block the transmission of pain impulses $[\boldsymbol{V}]$
b. “codeine crosses blood-brain barrier more easily»
morphine has more hydroxyl/OH «groups than codeine» $[\boldsymbol{U}]$
codeine/ether group is less polar
OR
hydroxyl/OH «groups in morphine» $\mathrm{H}$-bond to water $[\boldsymbol{V}]$
Note: Award [1 $\max$ ] if no statement or an incorrect statement about the blood-brain barrier.
Question
Medicines and drugs are tested for effectiveness and safety.
a. Distinguish between therapeutic window and therapeutic index in humans.
Therapeutic window:
Therapeutic index:
$b(i)$ State one advantage of using morphine as an analgesic.
$\mathrm{b}$ (ii Explain why diamorphine (heroin) is more potent than morphine using section 37 of the data booklet.
▶️Answer/Explanation
Markscheme
a. Therapeutic window:
range of dosage «over which a drug» provides the therapeutic/desired effect without causing adverse/toxic effects [ $\checkmark$ ]
Therapeutic index:
toxic dose of drug for $50 \%$ of population divided by minimum effective dose for $50 \%$ of population
OR
$\frac{\operatorname{TD} 50}{\operatorname{ED} 50}[\boldsymbol{c}]$
Note: M1 may be scored from a correctly labelled diagram.
Accept “difference between ED50/minimum effective/therapeutic dose «for $50 \%$ of population» AND TD50/toxic dose «for 50\% of population»” for $M 1$.
Do not accept reference to lethal dose used in therapeutic index in animal studies.
b(i)blocks pain impulses/binds with «opioid» receptors in brain/CNS
OR
effective against strong pain
OR
sedate patients to reduce trauma [ $\boldsymbol{C}]$
Note: Accept “effective against pain after surgery/cancer/following serious injury”.
Accept “relieves anxiety/stress associated with severe/terminal illness”.
b(ii)morphine has «two» hydroxyl groups AND diamorphine has «two» ester/ethanoate/acetate groups
OR
molecule of diamorphine is less polar than morphine
OR
groups in morphine are replaced with less polar/non-polar groups in diamorphine [ $\boldsymbol{V}]$
«less polar molecules» cross the blood-brain barrier faster/more easily
OR
diamorphine is more soluble in non-polar environment of CNS/central nervous system than morphine [ $\checkmark$ ]
Note: Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “fats” for “lipid”.
Accept “heroin” for “diamorphine”.
Question
Opiates are strong analgesics.
a. Explain why diamorphine (heroin) crosses the blood-brain barrier more easily than morphine.
[2]
b. Describe the analgesic action of an opiate.
[1]
c. Outline the meaning of the bioavailability of a drug.
[1]
▶️Answer/Explanation
Markscheme
a. blood-brain barrier is hydrophobic/non-polar/made of lipids
morphine has hydroxyl/OH «groups»/is more polar $A N D$ diamorphine has ester/ethanoate/ $\mathrm{COCH}_3 /$ acetate «groups»/is less polar/is lipid soluble
Accept “fats” for “lipid”.
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “non-polar” for “less polar” in M2.
b. «temporarily» binds to «opioid» receptor sites in the brain/CNS
OR
«temporarily» suppresses pain impulses in/to the brain/CNS
c. fraction/proportion/percentage of «administered dosage» enters blood/plasma/circulation
Accept “fraction/proportion/percentage of “administered dosage» that reaches target “part of human body»”.
Question
Analgesics are used to relieve pain in the body. Aspirin and paracetamol (acetaminophen) are both mild analgesics.
The structures of the strong analgesics morphine and heroin (diamorphine) can be found in Table 20 of the Data Book
Compare how mild and strong analgesics relieve pain in the body.
Identify the amine functional group in the morphine molecule below by drawing a ring around it.
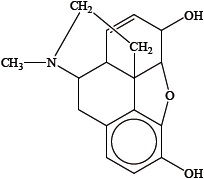
State the name of the functional group found in heroin but not in morphine.
State one advantage and one disadvantage of using morphine as a strong analgesic.
▶️Answer/Explanation
Markscheme
mild analgesics function by intercepting the pain stimulus at the source / interfere with the production of substances that cause pain/prostaglandins;
strong analgesics work by bonding to receptor sites in the brain / prevent the transmission of pain impulses without depressing the central nervous system;
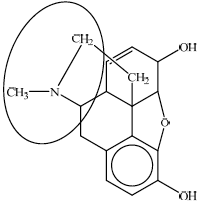
any circle around the nitrogen atom / the nitrogen atom and its three neighboring atoms;
ester;
Advantage: antidiarrheal/constipation (in treatment of diarrhea) / reduces coughing;
Disadvantage: addiction / tolerance / risk of overdose;
Examiners report
Most candidates were able to distinguish between the ways mild analgesics and strong analgesics relieve pain in part (b).
A substantial number of candidates failed to identify the tertiary amine in the structure of morphine. Candidates were inaccurate in drawing a circle around the amine group in part (c). Either just the nitrogen atom or nitrogen atom with its three neighbouring atoms should have been circled.
A large number of candidates confused the ester with an ether or carbonyl group as the functional group found in heroin but not in morphine.
Most candidates recognized the disadvantage of using morphine but they had extreme difficulty in stating a specific advantage for using morphine as a strong analgesic.
Question
Ethanol, a depressant, is sufficiently volatile to pass into the lungs from the bloodstream. The roadside breathalyser test uses acidified potassium dichromate(VI) which reacts with any ethanol present in the breath and converts it to ethanoic acid.
State the oxidation and reduction half-equations that occur in the breathalyser when ethanol is present in the breath.
Oxidation:
Reduction:
Describe the colour change that occurs to the acidified dichromate(VI) if ethanol is present in the breath.
Police use the intoximeter, an infrared spectrophotometer to confirm a roadside breathalyser test. Explain how the amount of ethanol is determined from the infrared spectrum.
▶️Answer/Explanation
Markscheme
Oxidation:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {\text{4}}{{\text{H}}^ + } + {\text{4}}{{\text{e}}^ – }\);
Reduction:
\({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 – } + {\text{14}}{{\text{H}}^ + } + {\text{6}}{{\text{e}}^ – } \to {\text{2C}}{{\text{r}}^{3 + }} + {\text{7}}{{\text{H}}_{\text{2}}}{\text{O}}\);
Accept balanced equation with molecular formulas.
If both equations are wrong, award [1] for C2H5OH \( \to \) CH3COOH and Cr2O72– \( \to \) 2Cr3+.
If correct equations are used but oxidation and reduction reversed, award [1].
orange to green;
peak at \({\text{2950 c}}{{\text{m}}^{ – 1}}\) / absorption occurs due to C–H bonds in ethanol;
No mark for absorption due to just ethanol, or O–H bond in ethanol (water vapour in breath also contributes).
intensity / height of peak / absorption / amount of transmittance depends on amount of ethanol / compare absorption to standard / reference/control sample / sample containing no alcohol;
Examiners report
Very few candidates gave correct equations for oxidation and reduction in a breath analyzer.
Most candidates described correctly the color change.
The way how a breath analyzer works seemed clear for most candidates.
Question
Aspirin, paracetamol (acetaminophen), morphine and diamorphine (heroin) are all pain killers. Their structures are given in Table 20 of the Data Booklet.
Aspirin is thought to interfere with the production of prostaglandins. Explain how this produces an analgesic effect.
Explain how morphine can prevent pain.
Paracetamol (acetaminophen) is generally considered to be safe to use as an analgesic in small doses. Other than the possibility of death, outline the problems associated with taking larger doses of paracetamol.
State one important use for aspirin other than the relief of pain and fever.
Explain what is meant by the term tolerance and suggest why this is a particular problem for heroin users.
▶️Answer/Explanation
Markscheme
prostaglandins are involved in the transmission of pain impulses (to the brain) / OWTTE;
morphine (temporarily) bonds to/inhibits receptor sites in the brain (without depressing central nervous system) / OWTTE;
causes blood disorders;
causes damage to kidney;
causes damage to liver;
causes damage to brain;
preventing (recurrence of) heart attacks/strokes / reduces blood clotting / thins the blood / anti-inflammatory;
tolerance: more of the drug needs to be taken to achieve the initial effect / OWTTE;
in order to achieve the desired effect heroin users may reach/exceed the lethal dose / heroin users are more likely to commit crimes to pay for gradually increasing doses of the drug / OWTTE;
Examiners report
This question was generally answered very well compared with other sections of the paper. In part (a) most candidates knew that prostaglandins is involved in the transmission of pain impulses to the brain.
In part (b) there was some confusion between signals and receptors when describing how morphine can prevent pain.
In part (c) most candidates could outline problems associated with larger doses of paracetamol (acetaminophen), although some candidates confused aspirin and paracetamol and incorrectly referred to Reye’s syndrome or stomach bleeding.
Most candidates stated a use for aspirin other than relief of pain or fever in part (d).
In part (f) most candidates successfully stated the meaning of tolerance and suggested why it is a particular problem for heroin users.
Question
State the differences between the structures of morphine and diamorphine (heroin). State the names of all functional groups in the molecule of morphine.
Differences:
Functional groups:
▶️Answer/Explanation
Markscheme
Differences:
(two) hydroxy(l)/alcohol and phenol groups are esterified/replaced with ester/ethanoate/acet(yl)oxy groups / OWTTE;
Accept formulas instead of group names.
Functional groups:
hydroxy(l)/alcohol/phenol;
ether/oxa;
(tertiary) amine/amino;
double bond/alkene;
aromatic/benzene ring/phenyl/aryl;
Examiners report
Many candidates were able to identify the differences in the structures of morphine and diamorphine, but in the second part of the question quite a number of candidates gave the formula of the functional groups rather than their names, as required by the question.
Question
Mild analgesics such as aspirin, and strong analgesics such as opiates, differ not only in their potency but also in the ways they act on the central nervous system.
(a) Describe how mild and strong analgesics provide pain relief.
Mild analgesics:
Strong analgesics:
(b) Discuss two advantages and two disadvantages of using morphine and other opiates for pain relief.
Advantages:
Disadvantages:
▶️Answer/Explanation
Markscheme
(a) Mild analgesics:
suppress the production of prostaglandins/pain-sensitizing substances / intercept the pain stimulus at the source;
Strong analgesics:
bind to (opioid) receptors in the CNS/central nervous system/brain / suppress the transmission of pain impulses to the brain / OWTTE;
(b) Advantages: [2 max]
strong(er) analgesics / relieve acute/extreme pain;
wide therapeutic window / OWTTE;
relieve anxiety / induce relaxation / improve the quality of life;
intravenous/faster distribution of drug;
Disadvantages: [2 max]
euphoria / lack of self-control / dangerous behaviour;
addiction/dependence / withdrawal symptoms;
tolerance / increased risk of overdose upon prolonged use;
kidney/renal failure;
risks associated with intravenous drug administration;
Accept other side-effects (including drug-specific for different opiates).
Examiners report
Many candidates displayed good knowledge about the differences in the mode of action of weak and strong analgesics, as well as the advantages and disadvantages of the latter.
Question
Aspirin and paracetamol (acetaminophen) are mild analgesics.
Morphine is a strong analgesic which is administered parenterally.
Explain why it is dangerous to take aspirin when ethanol has also been consumed.
State the meaning of the term parenteral.
Explain how a strong analgesic such as morphine prevents pain.
The structures of morphine and diamorphine (heroin) are shown in Table 20 of the Data Booklet. State the name of a functional group present in diamorphine (heroin) but not in morphine.
▶️Answer/Explanation
Markscheme
increased risk of stomach bleeding;
administered by injection;
(temporarily) bond to receptor sites in the brain/CNS;
prevent the transmission of pain impulses;
ester;
Examiners report
Most candidates were very familiar with analgesics and the synergistic effect of ethanol and aspirin.
Many of the weaker candidates thought that parenteral administration of morphine required supervision by parents or authorities. Assessment statement D.1.3 outlines the meaning of this technique.
Many candidates gave good descriptions of how morphine prevents pain.
[N/A]
Question
Analgesics can be either mild or strong.
Morphine, codeine and diamorphine (heroin) are all examples of strong analgesics. Their structures are found in Table 20 of the Data Booklet.
Explain how mild and strong analgesics prevent pain.
Mild analgesics:
Strong analgesics:
State a reason why it is dangerous to use aspirin while consuming alcohol.
Deduce from the structures the names of two functional groups present in all three analgesics.
Deduce the name of one functional group present in diamorphine (heroin) but not in morphine or codeine.
▶️Answer/Explanation
Markscheme
Mild analgesics:
intercept pain stimulus at source / inhibit release of substances/prostaglandins that cause pain/swelling/fever / OWTTE;
Strong analgesics:
(temporarily) bond to receptor sites in brain / prevent transmission of pain impulses in central nervous system/CNS / OWTTE;
Award [1 max] if states that mild analgesics act at source and strong analgesics act at brain/CNS.
increased risk of stomach bleeding;
Any two for [1]
amine
benzene ring
alkene
ether
Allow benzene or phenyl (group) but not arene.
ester;
Names of functional groups must be stated for both marks in part (d).
Do not apply ECF in (d) if formulas (for example, C=C) given instead of names.
Examiners report
The mode of action of mild and strong analgesics in part (a) was well known by most candidates; however, the terminology used was sometimes inaccurate and reflected confusion. For example, some candidates talked about intercepting pain receptors on site of injury for mild analgesics.
Part (c) was well answered by the majority of candidates (increased risk of stomach bleeding when alcohol is consumed with aspirin), and two-thirds of the candidates identified the functional groups correctly in part (d). A common mistake was using the term esther which was not clear whether it was meant to indicate ether or ester.
Question
The structures of aspirin and diamorphine (heroin) are given in Table 20 of the Data Booklet.
Other than the benzene (aromatic) ring, state the name of the functional group that is common to both aspirin and diamorphine.
Describe the different ways in which aspirin and diamorphine function when they relieve or prevent pain.
Aspirin:
Diamorphine:
Other than the prevention of pain and/or the reduction of fever, state one reason why aspirin is often prescribed or recommended to some people for daily use.
Discuss one advantage and one disadvantage of taking diamorphine rather than morphine to relieve pain.
Advantage:
Disadvantage:
▶️Answer/Explanation
Markscheme
ester;
Accept ethanoate/acetate.
Do not accept formula.
Aspirin:
Intercepts pain stimulus at source / blocks/interferes with production of substances/prostaglandins that cause pain/swelling/fever / OWTTE;
Diamorphine:
(temporarily) bonds to/blocks/interferes with receptor sites/synapses in the brain / prevent transmission of pain impulses (without depressing central nervous system/CNS) / OWTTE;
Award [1 max] if answer states that mild analgesic acts at source and strong analgesics act in the brain/CNS.
prevent stroke/heart attack/disease / thin blood / reduces risk of blood clots / antiinflammatory;
Advantage:
stronger pain killer / lower dose required / quicker acting;
Disadvantage:
more addictive / easier to build up tolerance and exceed lethal dose / smaller therapeutic window/index / OWTTE;
Examiners report
This was well answered. Most students could correctly identify the group common to both analgesics and could explain the differences in their modes of action, though the terms used often lacked precision. The reasons for regularly taking low doses of aspirin and the advantages and disadvantages of morphine and heroin, were also well known.
Question
The development of new and improved medications for the reduction and management of pain is an important part of 21st-century medicine.
Explain the way that mild and strong analgesics prevent pain.
Mild analgesics:
Strong analgesics:
The structure of morphine and diamorphine (heroin) are shown in Table 20 of the Data Booklet. State the name of the functional group present in diamorphine that is not present in morphine.
Discuss two advantages and two disadvantages of the medical use of morphine and its derivatives.
Advantages:
Disadvantages:
▶️Answer/Explanation
Markscheme
Mild analgesics:
mild analgesics work by intercepting pain stimulus at source;
suppress production of prostaglandins/pain sensitizing substances / OWTTE;
Strong analgesics:
strong analgesics work directly on opioid/pain receptors in brain;
suppress transmission of pain impulses in brain/CNS / OWTTE;
ester;
Accept alkanoate/ethanoate/acetoxy.
Advantages:
Award [1 max] for any two of:
strong pain relief / strong analgesic;
sedation / OWTTE;
treatment of diarrhoea;
relieve coughing;
Disadvantages:
Award [1 max] for any two of:
addiction;
tolerance;
dependence;
constipation;
depresses respiratory drive;
Accept “criminals/drug addicts might get access to strong analgesics intended for medical use” / OWTTE.
Award [1 max] if one advantage and one disadvantage are given.
Examiners report
Although the question on mild and strong analgesics, (a), is a question that has been asked previously a myriad of times, few surprisingly scored all four marks. Candidates occasionally discussed types of medication rather than mode of action. For mild analgesics many did not state the fact that these analgesics work by intercepting the pain stimulus at the source itself. The suppression of the production of prostaglandins often was not alluded to. For strong analgesics the most common mistake involved candidates not referring to opioid receptors in the brain.
(b) proved no problem for candidates though some stated incorrect functional groups or classes (alcohol and carboxylic acid were common incorrect answers). Please note that to prepare new candidates for the 2016 syllabus, the markscheme was later altered to include the correct naming of functional groups following IUPAC guidelines.
In (c), most candidates scored at least one mark. For the advantage few stated the fact that morphine is a strong analgesic.
Question
The properties of four analgesics are summarized below.
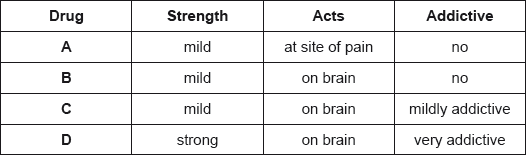
Deduce which drugs could be morphine, aspirin and codeine.
Morphine:
Aspirin:
Codeine:
Compare the structures of diamorphine (heroin) and morphine. Their structures are given in table 20 of the data booklet.
Two similarities:
One difference:
▶️Answer/Explanation
Markscheme
Morphine: D;
Aspirin: A;
Codeine: C;
Award [2] for all three correct, [1] for two correct.
Similarities:
Award [1 max] for any two:
benzene ring/aromatic ring/−C6H2;
Accept “phenyl” or “arene” but not C6H5– or benzene/C6H6.
(tertiary) amino/−NRR’/NRR’R”;
Accept “(tertiary) amine”.
carbon−carbon double bond/C=C;
Accept “alkene” or “alkenyl”.
ether/C−O−C;
Accept “both have the same ring structure / OWTTE”.
Difference:
ester/CH3COO in diamorphine/heroin and hydroxyl/OH in morphine;
Accept “ethanoate” for ester.
Accept “alcohol” or “hydroxy” for hydroxyl but not hydroxide.
Examiners report
Most candidates matched the analgesics to the properties successfully.
Most candidates identified two similarities and one difference between diamorphine and morphine. For the difference, it was necessary to mention both the hydroxyl groups in morphine and the ester groups in diamorphine.
Question
Morphine and its derivatives work by temporarily bonding to receptor sites in the brain, preventing the transmission of pain impulses.
Discuss one advantage and two disadvantages of using morphine as an analgesic.
Advantage:
Disadvantages:
The structures of morphine and diamorphine (heroin) are shown in table 20 of the data booklet. Describe the difference in the two structures by naming the functional groups.
▶️Answer/Explanation
Markscheme
Advantage:
relieves strong/severe pain / relieves pain caused by serious injury/cancer/surgery/heart attack / (intravenous so) faster acting;
Accept relieves anxiety / wide safety margin.
Do not accept just “relieves pain”.
Disadvantages:
Any two for [2 max] of:
(profound) tolerance develops;
addictive/(physical) dependence/habit forming;
causes drowsiness / mental clouding / depression/mood changes / constipation / loss of appetite / depression of the respiratory centre / nausea / suppresses cough reflex / pupil (of the eye) constriction / kidney/liver disorders;
Do not accept other disadvantages, such as overdose and coma.
morphine has (two) hydroxyl groups and diamorphine (heroin) has (two) ester groups;
Accept alcohol or hydroxy for hydroxyl but not hydroxide.
Examiners report
Many candidates focused their response on the description of morphine as a strong analgesic rather than on the advantage of using morphine although the disadvantages of its use were well known. The structural differences between morphine and diamorphine were often well known.
Question
Opiates have been used for thousands of years to alleviate pain. The structures of opiates are found in section 37 of the data booklet.
Diamorphine (heroin) can be synthesized from morphine. Identify the reagent necessary for this reaction and the by-product of this reaction.
The reaction can be monitored by infrared spectroscopy. Using section 26 of the data booklet, identify two IR absorbance ranges that would help distinguishing the two compounds.
Present in morphine but not in diamorphine:
Present in diamorphine but not in morphine:
Discuss how the differences in structure between morphine and diamorphine affect their absorption in the body.
▶️Answer/Explanation
Markscheme
Accept names or structural formulas for reagent and by-product.
Accept IUPAC or alternative names of compounds e.g. acetic acid.
Award M2 only if the by-product corresponds to the reagent.
Present in morphine but not in diamorphine:
«has OH and absorbance at» 3200–3600 «cm–1»
Present in diamorphine but not in morphine:
«has C=O and absorbance at» 1700–1750 «cm–1»
morphine has «two» hydroxyl «groups» AND diamorphine/heroin has «two»
ester/ethanoate/acetate «groups»
morphine is more polar than diamorphine/heroin
diamorphine/heroin crosses the blood-brain barrier «easily»
morphine is soluble in blood «plasma»
OR
diamorphine/heroin is «more» soluble in lipids
Accept converse argument throughout. Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Do not accept “diamorphine/heroin is non-polar” for M2.
Question
Methadone, a synthetic opioid, binds to opioid receptors in the brain.
Compare and contrast the functional groups present in methadone and diamorphine (heroin), giving their names. Use section 37 of the data booklet.
Methadone is sometimes used to help reduce withdrawal symptoms in the treatment of heroin addiction. Outline one withdrawal symptom that an addict may experience.
▶️Answer/Explanation
Markscheme
Similarity:
both contain «at least one» benzene/aromatic ring
OR
both contain amino «group»
Difference:
diamorphine has one benzene/aromatic ring AND methadone has two phenyl «groups»
OR
diamorphine has one vinylene/ethenylene/1,2-ethenediyl «group» AND methadone has no vinylene/ethenylene/1,2-ethenediyl «group»
OR
diamorphine has one ether «group» AND methadone has no ether «group»
OR
diamorphine has «two» ethanoate/acetate «groups» AND methadone has no ethanoate/acetate «groups»
Accept “both contain carbonyl «groups»”.
Accept “amine” for “amino «group»”.
Accept “phenyl” for “benzene ring” in M1 and M2 although there are no phenyl groups in diamorphine, as the benzene ring in this compound is a part of a polycyclic structure.
Do not accept “arene” or “benzene” alone in M1 and M2.
Accept “alkenyl/alkene” for “vinylene/ethenylene/1,2-ethenediyl” and “ester” for “ethanoate/acetate”.
Accept “methadone has a ketone/carbonyl AND diamorphine does not/has an ester/ethanoate/acetate”.
Accept “diamorphine is a heterocycle/heterocyclic compound AND methadone is not a heterocycle/heterocyclic compound”.
feeling depressed/anxious/irritable
OR
craving for opioids/heroin
OR
experience fever/cold sweats/nausea/vomiting/insomnia/muscle pain/cramps/diarrhea/increased rate of respiration/increased heartbeat/lacrimation
Accept listed symptoms (eg, depression, anxiety, fever etc.).
Some of the most common symptoms are listed here – there may be other valid ones. Accept “headaches”.
Question
Medicines have a variety of different effects on the body and act at the molecular level.
Morphine and codeine are strong analgesics. Their structures are given in section 37 of the data booklet.
Dose response curves are determined for each drug.
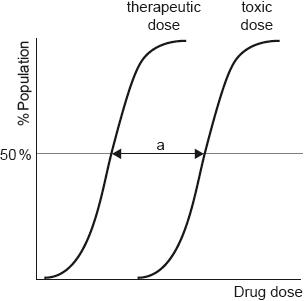
Outline the significance of range “a”.
Suggest the type of reaction used to convert morphine to codeine.
State and explain the action of opiates as painkillers.
▶️Answer/Explanation
Markscheme
«measures» therapeutic window/margin «of a drug»
OR
range of doses that produce a therapeutic effect without causing toxic effects
Accept “difference between ED50/minimum effective/therapeutic dose «for 50% of population» AND TD50 /toxic dose «for 50% of population»”.
Do not accept “therapeutic index”.
Do not accept “lethal/fatal dose” as this is not LD50.
[1 mark]
«nucleophilic» substitution/SN
Accept “methylation”.
[1 mark]
work directly on opioid/pain receptors «in brain»
suppress pain impulses in brain/CNS
resemble endorphins/enkephalins/natural chemical painkillers «produced in the brain and spinal cord»
Do not award mark for “resemble hormones”.
[2 marks]
Question
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
Suggest a reagent used to prepare diamorphine from morphine.
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
▶️Answer/Explanation
Markscheme
Any two of:
diamorphine has ester/ethanoate/acetate «groups» AND morphine has hydroxyl «groups»
diamorphine/ester/ethanoate/acetate groups less polar
diamorphine more soluble in lipids
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “diamorphine non-polar”.
Accept converse statements.
[2 marks]
ethanoic/acetic anhydride
OR
ethanoyl/acetyl chloride
Accept other possible reagents, such as ethanoic/acetic acid or acetyl bromide.
Accept chemical formulas.
[1 mark]
morphine has a smaller therapeutic window
Accept converse statements.
Accept “codeine has lower activity” OR “codeine has lower risk of overdose” OR “codeine is less potent”.
Do not accept “lower abuse potential for codeine” OR “codeine less addictive” OR “codeine has a lower bioavailability”.
[1 mark]
Question
Some analgesics are derived from compounds found in plants.
Aspirin is a mild analgesic derived from salicylic acid found in willow bark.
Describe how mild analgesics function.
The strong analgesics morphine and codeine are opiates. Outline how codeine can be synthesized from morphine. The structures of morphine and codeine are in section 37 of the data booklet.
Explain why opiates are addictive.
▶️Answer/Explanation
Markscheme
prevents/interferes with the production of prostaglandins
OR
prevents/interferes with the production of substances responsible for
inflammation/pain/fever
at the site of injury/source of pain
react with CH3I/methyl iodide «in alkaline solution»
Accept “react with CH3Cl/methyl chloride” OR “react with methyl halide”.
Accept name or formula of a suitable specific methylating reagent (eg trimethylphenylammonium chloride etc.).
Accept “hydroxy/alcohol” but not “hydroxide” for “hydroxyl”.
Any two of:
interact with opioid receptors in the brain
alter the structure of brain cells
OR
alter the way the brain works «so that it only works normally when the opiates are present»
OR
prevents transmission of pain impulses inside the brain
release dopamine «that the person craves»
OR
give a feeling of pleasure/euphoria «that the person craves»
withdrawal symptoms «prevent patient from terminating drug use»
Accept specific withdrawal symptoms.
[Max 2 Marks]
Question
Many drugs, including aspirin, penicillin, codeine and taxol, have been modified from compounds that occur naturally.
Aspirin is often taken to reduce pain, swelling or fever. State one other use of aspirin.
State what is meant by the bioavailability of a drug.
Outline how the bioavailability of aspirin may be increased.
Compare and contrast the IR spectrum of aspirin with that of salicylic acid, using section 26 of the data booklet.
Describe how penicillin combats bacterial infections.
Outline two consequences of prescribing antibiotics such as penicillin unnecessarily.
State how penicillins may be modified to increase their effectiveness.
Morphine and codeine are strong analgesics. Outline how strong analgesics function.
Suggest one reason why codeine is more widely used than morphine as an analgesic.
▶️Answer/Explanation
Markscheme
Any one of:
anticoagulant
lower risk of heart attack/strokes
prevent recurrence of heart attack/stroke
prevents cancer of colon/oesophagus/stomach
Accept “prevents/reduces blood clots” OR “blood thinner”.
[1 mark]
fraction/proportion/percentage «of administered dosage» that reaches target «part of human body»
OR
fraction/ proportion/percentage «of administered dosage» that reaches blood «plasma»/systemic circulation
Accept “the ability of the drug to be absorbed by the body” OR “the extent to which the drug is absorbed by the body”.
Do not accept “the amount/quantity of the drug absorbed”.
[1 mark]
«intravenous» injection/IV
Accept “parenterally”.
Accept “react with alkali/NaOH” OR “convert to ionic form/salt”.
[1 mark]
One absorption found in both spectra:
Any one of:
1050–1410 cm–1 «C–O in alcohols, esters, ethers»
1700–1750 cm–1 «C=O in carboxylic acids, esters»
2500–3000 cm–1 «O–H in carboxylic acids»
2850–3090 cm–1 «C–H in alkanes, alkenes, arenes»
One absorption found in only one of the spectra:
3200–3600 cm–1 «O–H in alcohols, phenols»
Award [1 max] if candidate states bonds (C=O in both, O–H in salicylic acid only) but doesn’t quote wavelength ranges.
Accept a second/additional absorption at 1700–1750 cm–1 from the C=O in ester.
[2 marks]
Any two of:
ring is «sterically» strained
OR
ring breaks up/opens/reacts «easily»
OR
amide/amido group «in ring» is «highly» reactive
«irreversibly» binds/bonds to enzyme/transpeptidase
OR
inhibits enzyme/transpeptidase «in bacteria» that produces cell walls
OR
prevents cross-linking of bacterial cell walls
cells absorb water AND burst
OR
cells cannot reproduce
Award [1 max] for “interferes with cell wall production”.
Do not accept “cell membrane” instead of “cell wall”.
[2 marks]
Any two of:
leads to «bacterial» resistance/proportion of resistant bacteria increases
OR
leads to penicillinase-producing bacteria
damage to/contamination of bodies of water/ecosystems
destroys useful/beneficial bacteria
destroyed bacteria replaced by more harmful bacteria
Accept “endocrine disruptor”.
Do not accept “increased cost of developing antibiotics”.
[2 marks]
modify side chain
[1 mark]
temporarily bind to/block/interfere with receptor sites in brain
OR
prevent transmission of pain impulses within CNS/central nervous system
[1 mark]
codeine has a wider therapeutic window
Accept “codeine has lower activity” OR “codeine has lower risk of overdose” OR “codeine is less potent” OR “codeine has less side-effects”.
Do not accept “lower abuse potential for codeine” OR “less addictive «than morphine»” OR “codeine has a lower bioavailability” OR “available without prescription” OR “cheaper”.
[1 mark]
Question
Morphine and diamorphine (heroin) are both opioids.
Explain why diamorphine is more potent than morphine using section 37 of the data booklet.
▶️Answer/Explanation
Markscheme
morphine has hydroxyl/OH groups/is more polar AND diamorphine has ester/ethanoate/acetate groups/is less polar/is lipid soluble
crossing blood brain barrier is easier for non-polar/less polar compounds/for lipid soluble compounds
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “fats” for “lipid”.
[2 marks]

