Question
Describe the original source of Taxol and the disadvantages of obtaining the medication from this source.
Answer/Explanation
Answer:
bark of yew tree
kills tree
OR
tree grows slowly
OR
low yield
Question
The chemotherapy drug Taxol is now manufactured using a chiral auxiliary and 10-deacetybaccatin III instead of the bark of Pacific yew trees.
(a) A chiral auxiliary used in the synthesis of Taxol is trans-2-phenylcyclohexanol. Deduce and label with asterisks (*) the positions of the two chiral carbon atoms in the molecule.
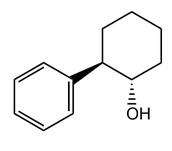
(b) State one natural source of 10-deacetylbaccatin III now used to synthesize Taxol.
(c) State two principles of green (sustainable) chemistry in drug manufacturing, other than using less hazardous or toxic reactants.
Answer/Explanation
Answer:
(a) 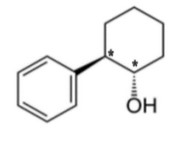
one for each carbon correctly identified
(b) needles «of European» yew «tree»
(c) Any two of:
energy efficiency
prevention of waste/recycling
atom economy/more efficient processes
safer/reduced use of solvents
design for degradation
more sustainable sourcing of reactants/feedstock
Question
Consider the structures of medicinal molecules in section 37 of the data booklet.
a. Explain how zanamivir works as a preventative agent against flu viruses.
b(i)Circle the side-chain in penicillin on the structure below.
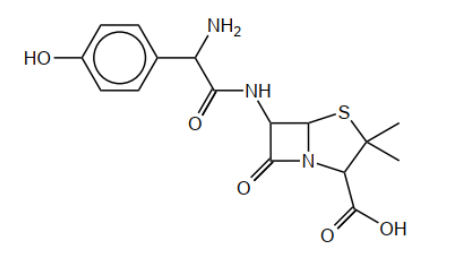
b(iiExplain, with reference to the action of penicillin, why new penicillins with different side-chains need to be produced.
c(i)State and explain the relative solubility of codeine in water compared to morphine and diamorphine.
c(ii)State the natural source from which codeine, morphine and diamorphine are obtained.
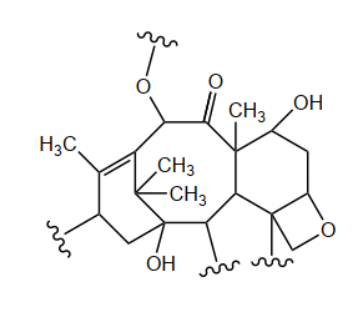
d. Circle two chiral carbons in the section of the Taxol structure below.
▶️Answer/Explanation
Markscheme
a. «drug» blocks/inhibits «viral» enzyme/neuraminidase/NA «activity» prevents virus from leaving/escaping host cells «thus cannot infect other cells»
Do not accept other anti-viral methods (as question is specific to Zanamivir).
b(i).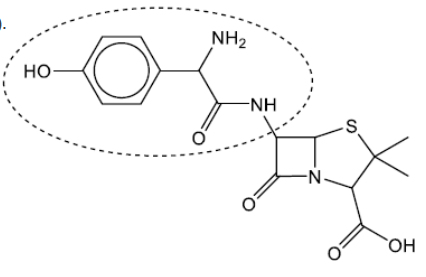
Accept a circle that does not surround the amido group.
Do not accept a circle that only surrounds the phenol group.
b(iipacterial resistance «to older penicillins/antibiotics»
prevent penicillinase/beta-lactamase/enzyme in bacterium to deactivate/open penicillin/beta-lactam ring
Accept “antibiotic resistant bacteria” but not “antibiotic resistance” for M1.
Accept “reduce allergic reactions from penicillin” for M2.
Award [1 max] for “increased efficiency” OR “increased stability in GIT”.
Do not accept “bacteria develop tolerance”.
c(i)codeine less soluble «in water» than morphine $\boldsymbol{A N D}$ more soluble than diamorphine
OR
morphine > codeine > diamorphine «in terms of solubility in water»
more/stronger/greater hydrogen/H bonding «due to more hydroxyl groups leads to greater solubility»
c(ii)pium poppy/plants/seeds
Accept “poppy” OR “opioid”.
d.
\text { any two chiral carbons identified }
