Question
The production of many pharmaceutical drugs involves the use of solvents.
(a) State one problem associated with chlorinated organic solvents as chemical waste.
(b) Suggest how the principles of green chemistry can be used to overcome the environmental problems caused by organic solvents.
Answer/Explanation
Answer:
(a) Any one of:
«most are» toxic «to living organisms»
OR
incomplete combustion/incineration can produce toxic products/dioxins/phosgene
OR
carcinogenic
«some can be» greenhouse gases
ozone-depleting
can contribute to formation of «photochemical» smog
accumulate in groundwater
OR
have limited biodegradability
cost/hazards of disposal
(b) use solvent-free synthetic methods
OR
use water/supercritical carbon dioxide/non-toxic/low-toxic/biodegradable
compounds as a solvent
OR
recover/reuse solvents
OR
use a non-chlorinated solvent
Question
Analytical chemistry is very important in drug detection.
(a) An example of a steroid is testosterone.
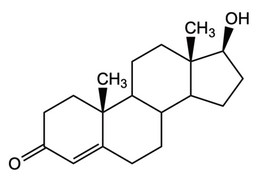
(i) State the technique used to separate steroids, such as testosterone, from biological fluids.
Once separation has been completed, the components can be identified using mass spectrometry. The following mass spectrum is of testosterone:
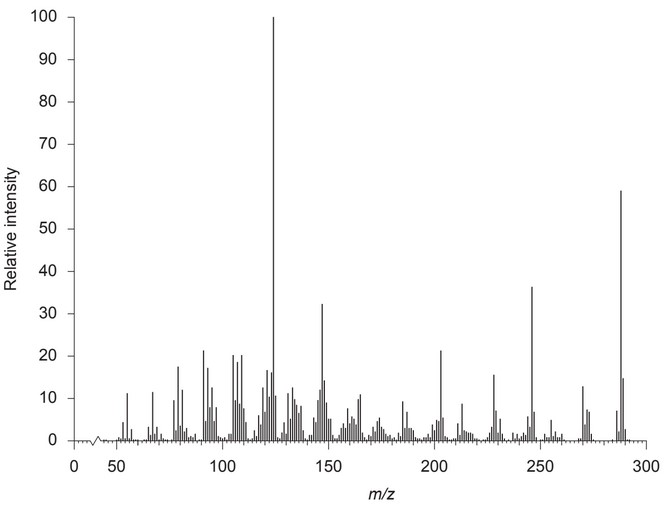
(ii) Explain how the fragments at m/z 288 and 273 can be used to show that it is testosterone, \(C_{19}H_{28}O_2\), \(M_r\) 288.
m/z 288:
m/z 273:
(b) One breathalyser technique is to measure the change in colour when the dichromate ion is reduced to the chromium(III) ion:
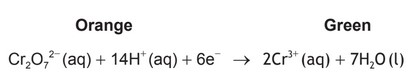
Deduce the half-equation for the oxidation of ethanol and the overall redox equation occurring in the breathalyser.
Half-equation for oxidation of ethanol:
Overall balanced redox equation:
Answer/Explanation
Answer:
(a) (i) chromatography
(ii) m/z 288:
molecular ion/\(M^+\)/\(C_{19}H_{28}O_2^+\)
m/z 273:
\(C_{18}H_{25}O_2^+\)
OR
loss of methyl/\(CH_3\) group” or “\((M – CH_3)^+\)
(b) 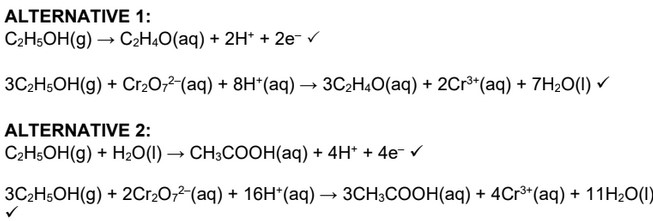
Question
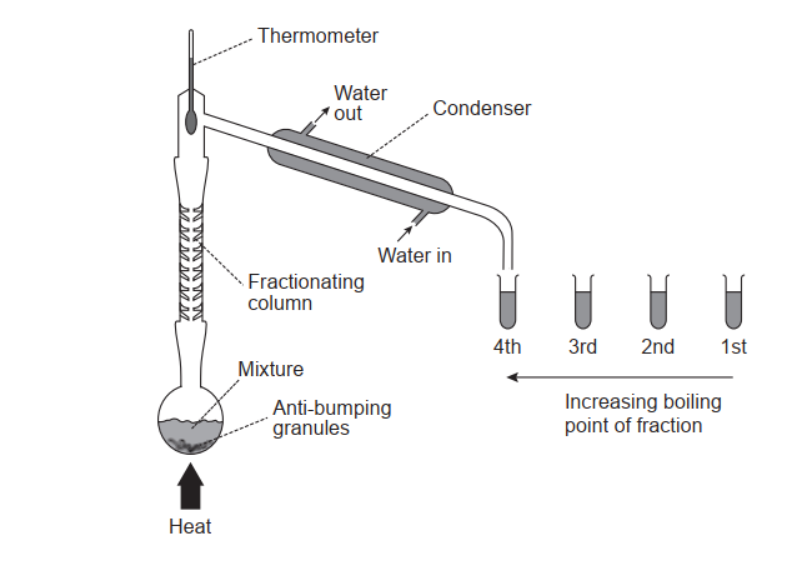
A mixture of $0.100 \mathrm{~mol}$ ethanal, $0.100 \mathrm{~mol}$ ethanol and $0.200 \mathrm{~mol}$ ethanoic acid is fractionally distilled.
a(i)Calculate the mole fraction of ethanal in the mixture.
a(ii)the vapour pressure of pure ethanal at $20^{\circ} \mathrm{C}$ is $101 \mathrm{kPa}$.
Calculate the vapour pressure of ethanal above the liquid mixture at $20^{\circ} \mathrm{C}$.
b. Describe how this mixture is separated by fractional distillation.
▶️Answer/Explanation
Markscheme
$$
\mathrm{a}(\mathrm{i}) \ll \chi_{\text {ethanal }}=\frac{0.100}{0.100+0.100+0.200}=» 0.250 \vee
$$
Accept “25\%”.
$$
\mathrm{a}(\mathrm{ii}) \ll \rho_{\text {ethanal }}=0.250 \times 101=» 25.3 \ll \mathrm{kPa} »
$$
b. Any two of:
continuous evaporation and condensation
OR
increased surface area in column helps condensation
Accept “glass “beads» aid condensation «in fractionating column»”.
temperature decreases up the fractionating column
liquids condense at different heights
$O R$
liquid of lowest boiling point collected first
OR
liquid with weakest intermolecular forces collected first
OR
most volatile component collected first
$O R$
fractions/liquids collected in order of boiling point/volatility
Accept “liquids collected in order of molar mass”.
