Question
Which contains the most atoms of oxygen?
64 g of
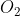
1.2 ×
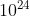 molecules of
molecules of 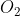
64 g of
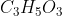
1.2 ×
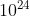 molecules of
molecules of 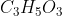
▶️Answer/Explanation
Ans: D
We need to check the number of atoms present one by one.
From Avogadro’s Number,
A)
1 mole of oxygen contains \(6.023 \times 10^{23}\)
32 gram of oxygen O2 = 1 mole
64 gram of oxygen = 2 mole
2 mole of of oxygen contains \(2 \times 6.023 \times 10^{23} = 12.046 × 10^{23} \) atoms
B)
1 molecule of oxygen O2 = 2 oxygen atoms
\(1.2 × 10^{24}\) molecules of oxygen O2 = \(2 \times 1.2 \times 10^{24} = 24 × 10^{23} \) oxygen atoms
C)
1 mole of
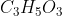 contains \(3 × 6.023 × 10^{23}\) oxygen atoms.
contains \(3 × 6.023 × 10^{23}\) oxygen atoms.89 grams of
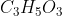 is 1 mole.
is 1 mole.64 gram of
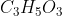 is 0.719 mole
is 0.719 mole0.719 mole of
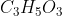 contains \(0.719 × 3 × 6.023 × 10^{23}\) =\( 12.991 × 10^23\) oxygen atoms.
contains \(0.719 × 3 × 6.023 × 10^{23}\) =\( 12.991 × 10^23\) oxygen atoms.D)
6.023 × 10^23 molecules = 1 mole of
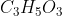
1.2 × 10^24 molecule is 2 mole of
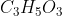
1 mole of
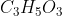 contains \(3 × 6.023 × 10^{23}\) oxygen atoms.
contains \(3 × 6.023 × 10^{23}\) oxygen atoms.2 mole of
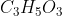 contains \(2 × 3 × 6.023 × 10^{23} = 36.138 × 10^{23}\) oxygen atoms.
contains \(2 × 3 × 6.023 × 10^{23} = 36.138 × 10^{23}\) oxygen atoms.Which is the maximum among these. Hence the correct option is D.
Question
Which coefficients would balance this equation?
__ \({\text{Mn}}{{\text{O}}_2} + \) __ \({\text{HCl}} \to \) __ \({\text{MnC}}{{\text{l}}_2} + \) __ \({\text{C}}{{\text{l}}_2} + \) __ \({{\text{H}}_2}{\text{O}}\)
Answer/Explanation
Ans: C
To balance the equation, the number of atoms in each element on the left is equal to the right.
A)
The oxygen atom is not balanced.
B)
The oxygen, hydrogen, and chlorine atoms are not balanced.
C)
Here, the number of atoms in each element on the left is equal to the right. So the correct answer is option C.
Question
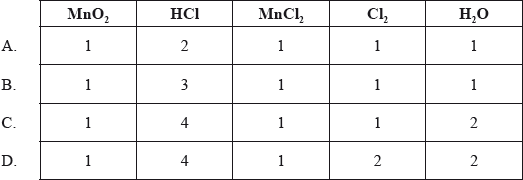
What are the coefficients of \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) and \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) when the following equation is balanced using the smallest possible whole numbers?
___ \({\text{C}}{{\text{a}}_3}{{\text{(P}}{{\text{O}}_4}{\text{)}}_2}{\text{(s)}} + \) ___ \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to \) ___ \({\text{CaS}}{{\text{O}}_3}{\text{(s)}} + \) ___ \({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\)
▶️Answer/Explanation
Ans: D
To balance the equation, the number of atoms in each element on the left is equal to the right.
From the coefficients of these two compounds, we can balance H atoms as it is present in these two compounds only.
A)
When the coefficients are 1 and 2, the number of hydrogen atoms in the reactants side are 2 and in the product side are 6. Hence, H atoms are not balanced.
B)
When the coefficients are 2 and 3, the number of hydrogen atoms in the reactants side are 4 and in the product side are 9. Hence, H atoms are not balanced.
C)
When the coefficients are 3 and 1, the number of hydrogen atoms in the reactants side are 6 and in the product side are 3. Hence, H atoms are not balanced.
D)
When the coefficients are 3 and 2, the number of hydrogen atoms in the reactants side are 6 and in the product side are 6. Hence, H atoms are now balanced.
Question
Which expression gives the sum of all the coefficients for the general equation for the complete
combustion of hydrocarbons?
___ \({{\text{C}}_x}{{\text{H}}_y}{\text{(g)}} + \) ___ \({{\text{O}}_{\text{2}}}{\text{(g)}} \to \) ___ \({\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + \) ___ \({{\text{H}}_{\text{2}}}{\text{O(l)}}\)
A. \(1 + x + \frac{y}{4}\)
B. \(1 + x + \frac{y}{2}\)
C. \(1 + 2x + \frac{{3y}}{4}\)
D. \(1 + 2x + \frac{{3y}}{2}\)
▶️Answer/Explanation
C
Coefficient of CxHy = 1
To balance C atoms, coefficient of CO2 = x
To balance H atoms, coefficient of H2O = y/2
Now balancing O atoms, coefficient of O2 = x + y/4
Now, sum of all coefficients = 1 + (x + y/4) + (x) + y/2 = 1 + 2x + 3y/4.
