Question
Smog is common in cities throughout the world. One component of smog is PAN (peroxyacylnitrate) which consists of 20.2% C, 11.4% N, 65.9% O and 2.50% H by mass. Determine the empirical formula of PAN, showing your working.
▶️Answer/Explanation
Markscheme
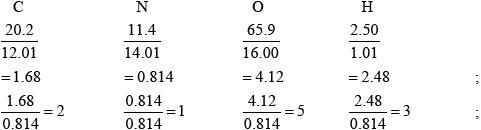
\({{\text{C}}_{\text{2}}}{\text{N}}{{\text{O}}_{\text{5}}}{{\text{H}}_{\text{3}}}\);
No penalty for use of 12, 1 and/or 14.
Award [1 max] if the empirical formula is correct, but no working shown.
Detailed Solution:
PAN components C:N:O:H
Mass ratio 20.2:11.4:65.9:2.5
Molar mass ratio 12:14:16:1
Moles ratio 1.67:0.814:4.12:2.5
i.e. C:N:O:H = 2:1:5:3
Hence, empirical formula of PAN is \({{\text{C}}_{\text{2}}}{\text{N}}{{\text{O}}_{\text{5}}}{{\text{H}}_{\text{3}}}\).
Question
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\xrightarrow{{reaction 3}}{\text{RCOOH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
The mechanism in reaction 2 is described as SN2.
Propan-1-ol has two structural isomers.
a.The alkane contains 81.7% by mass of carbon. Determine its empirical formula, showing your working.[3]
(ii) State the reagent(s) and conditions needed for reaction 3. [2]
(ii) Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and (iii)draw the structure of the transition state.[4]
(ii) Identify the isomer from part (f) (i) which has the higher boiling point and explain your choice. Refer to both isomers in your explanation.[4]
▶️Answer/Explanation
Markscheme
a.
\({{\text{n}}_{\text{C}}} = \frac{{81.7}}{{12.01}} = 6.80\) and \({{\text{n}}_{\text{H}}} = \frac{{18.3}}{{1.01}} = 18.1\);
ratio of 1: 2.67 /1: 2.7;
\({{\text{C}}_3}{{\text{H}}_8}\);
No penalty for using 12 and 1.
\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}\);
(i) \({\text{B}}{{\text{r}}_2}\) /bromine;
UV/ultraviolet light;
Accept hf/hv/sunlight.
(ii) \({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }\) / \({\text{MnO}}_4^ – \) and acidified/\({{\text{H}}^ + }\) /\({{\text{H}}_3}{{\text{O}}^ + }\);
Accept names.
heat / reflux;
i. Initiation:
\({\text{B}}{{\text{r}}_2} \to 2{\text{Br}} \bullet \);
ii. Propagation:
\({\text{Br}} \bullet + {\text{RC}}{{\text{H}}_3} \to {\text{HBr}} + {\text{RC}}{{\text{H}}_2} \bullet \);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{RC}}{{\text{H}}_2}{\text{Br}} + {\text{Br}} \bullet \);
iii. Termination:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{Br}} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{Br}}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{RC}}{{\text{H}}_2} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{R}}\);
Award [1] for any termination step.
Accept radical with or without \( \bullet \) throughout.
Do not penalise the use of an incorrect alkane in the mechanism.
(i) substitution and nucleophilic and bimolecular/two species in rate-determining step;
Allow second order in place of bimolecular.
(ii) 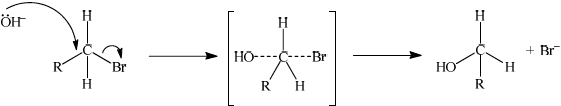
curly arrow going from lone pair/negative charge on O in OH– to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
iii. Representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180o to each other.
Do not award M3 if OH—-C bond is represented unless already penalised in M1.
Do not penalise the use of an incorrect alkyl chain in the mechanism.
(i) \({\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
\({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\);
Allow more detailed structural formulas.
(ii) \({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\) has higher boiling point due to hydrogen bonding;
\({\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) has lower boiling point due to Van der Waals’/London/dispersion/dipole-dipole forces;
Hydrogen bonds in \({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\) are stronger;
Allow ecf if wrong structures suggested.
Detailed Explanation:
a. Alkanes have C and H.
C =81.7% then H = 18.3%
Mass ratio C:H = 81.7:18.3
Molar ratio C:H = 6.81:18.3 = 1:2.7 = 3:8
Alkane is C3H8.
b. Given, Mass of CO2 = Mass of alkane.
Since they have equal volume at same temperature and pressure, they will also have equal number of moles.
Their molar mass will also be equal. The general formula for alkanes is CₙH₂ₙ₊₂,
Molar mass of alkane = 44 = 12n+2n+2 i.e. n=3
The required alkane is C3H8.
c.
i. In the bromination of alkyl (RCH₃) groups using UV light, a common reagent used is molecular bromine (Br₂).
ii. The reagent commonly used for the oxidation of alcohols to carboxylic acids is a strong oxidizing agent called potassium permanganate (KMnO₄) or a related oxidant such as chromium trioxide (CrO₃) or Jones reagent (a mixture of chromic acid and sulfuric acid).
d.
i. Initiation: The process begins with the initiation step, where the UV light provides the energy required to break the bromine (Br₂) molecule into two bromine radicals (Br•). This is represented as: Br₂ + UV light → 2 Br•
ii. Propagation: Once the bromine radicals are formed, they react with the alkyl group (RCH₃) in a series of propagation steps. In the case of bromination, there are two primary propagation steps:
\({\text{Br}} \bullet + {\text{RC}}{{\text{H}}_3} \to {\text{HBr}} + {\text{RC}}{{\text{H}}_2} \bullet \);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{RC}}{{\text{H}}_2}{\text{Br}} + {\text{Br}} \bullet \)
iii. Termination: The termination step involves the combination of two radical species, leading to the termination of the chain reaction. In bromination reactions, the termination steps can include:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{Br}} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{Br}}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{RC}}{{\text{H}}_2} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{R}}\)
e.
i. In the context of SN2 (substitution nucleophilic bimolecular) reactions,
S: Substitution The “S” represents the substitution step in the reaction, indicating that one group is being substituted by another.
N: Nucleophilic The “N” represents the nucleophilic species involved in the reaction. The nucleophile is an electron-rich species that donates a pair of electrons to form a new bond with the substrate.
2: Bimolecular The “2” indicates that the rate-determining step of the reaction involves the collision between two species: the nucleophile and the substrate (or electrophile). The reaction occurs in a single step where the nucleophile directly attacks the substrate while displacing a leaving group.
ii. The nucleophile, here represented as OH⁻, approaches the carbon atom attached to the bromine (C-Br) in the alkyl bromide. Simultaneously, the leaving group (Br⁻) starts to leave. This step is concerted, meaning that the bond between C and Br begins breaking as the new bond between C and OH forms. The nucleophile attacks the carbon atom, and the leaving group is displaced. The reaction can be represented as:

iii. The transition state represents the highest energy point along the reaction pathway. In an SN2 reaction, the transition state occurs when the nucleophile is partially bonded to the carbon atom, and the leaving group is partially detached. The transition state is characterized by a trigonal bipyramidal geometry around the carbon atom, with partial bond formation and partial bond cleavage. It is a short-lived, high-energy species that quickly leads to the formation of the products.
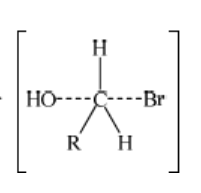
Here, OH and Br both are bonded partially.
f.
i. n-Propanol (1-Propanol):Structural Formula: CH₃-CH₂-CH₂OH
and Methoxyethane (Ethyl methyl ether): Structural Formula: CH₃O-CH₂-CH₃
ii. 1-Propanol (n-Propanol) has a higher boiling point compared to Ethyl methyl ether.
The boiling point of a compound is influenced by intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces. In the case of 1-Propanol, it contains an -OH group, which is capable of forming hydrogen bonds with neighboring molecules. Hydrogen bonding is a strong intermolecular force that requires more energy to break, resulting in a higher boiling point. Therefore, 1-Propanol exhibits stronger intermolecular forces due to hydrogen bonding.
On the other hand, Ethyl methyl ether lacks the -OH group and, therefore, does not have the ability to form hydrogen bonds. It primarily experiences dipole-dipole interactions and London dispersion forces. These intermolecular forces are generally weaker than hydrogen bonding, resulting in a lower boiling point for Ethyl methyl ether compared to 1-Propanol.
