Question
In a class experiment, students were asked to determine the value of $\mathbf{x}$ in the formula of a hydrated salt, $\mathrm{BaCl}_2 \cdot \mathrm{xH}_2 \mathrm{O}$. $\mathrm{They}^{\text {followed these instructions: }}$
1. Measure the mass of an empty crucible and lid.
2. Add approximately $2 \mathrm{~g}$ sample of hydrated barium chloride to the crucible and record the mass.
3. Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
4. After cooling, reweigh the crucible, lid and contents.
5. Repeat steps 3 and 4.
Their results in three trials were as follows:
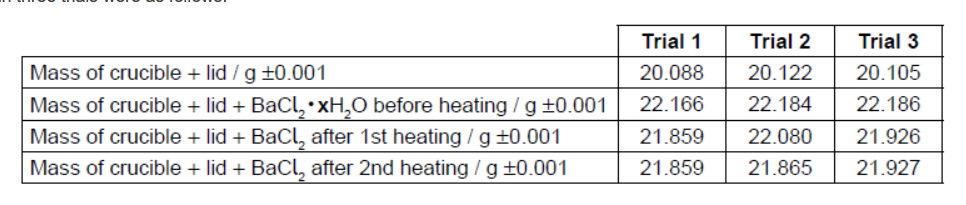
a. State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
b. In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for $\mathbf{x}$ in the hydrated salt.
c. List two assumptions made in this experiment.
▶️Answer/Explanation
Markscheme
a. repeat steps 3 and 4
OR
repeat step 5
OR
conduct a third heating
OR
«re»heat $\boldsymbol{A N D}$ «re»weigh
water still present
OR
need two consistent readings
OR
heat to constant mass
Accept “ensure even/strong heating” for M1.
Do not accept “cleaning/washing the crucible”.
b. soot/carbon deposited
OR
incomplete combustion
OR
air hole of Bunsen burner closed/not fully open
Accept “using a yellow “Bunsen burner» flame” for M1.
«value of $\mathbf{x}$ » lower
Only award M2 if M1 correct.
c. all mass loss is due to water loss
all the water «of crystallization» is lost
crucible does not absorb/lose water
crystal/ $\mathrm{BaCl}_2$ does not decompose/hydrolyse/oxidize/react with oxygen/air «when heated»
Accept “no loss of crystals/BaCl ${ }_2$ occurs”, “no impurities in the “weighed hydrated” salt”, “reaction goes to completion”, “heat was consistent/strong”, “crystal/BaCl $\mathrm{Bl}_2$ does not absorb water during cooling”, “balance has been calibrated” or “crucible was clean at the start”.
Do not accept “heat loss to surroundings” or “no carbon deposited on crucible”.
Reference to defects in apparatus not accepted.
Do not penalize if $\mathrm{BaCl}_2 \cdot \mathrm{xH}_2 \mathrm{O}$ is used for $\mathrm{BaCl}_2$.
Question
Water purity is often assessed by reference to its oxygen content.
The Winkler method uses redox reactions to find the concentration of oxygen in water. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of water was taken from a river and analysed using this method. The reactions taking place are summarized below.
\[\begin{array}{*{20}{l}} {{\text{Step 1}}}&{{\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ – }{\text{(aq)}} + {{\text{O}}_2}{\text{(aq)}} \to {\text{2Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 2}}}&{{\text{Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 3}}}&{{\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 – }{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} \to {{\text{S}}_4}{\text{O}}_6^{2 – }{\text{(aq)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}}} \end{array}\]
Outline the meaning of the term biochemical oxygen demand (BOD).
State what happened to the \({{\text{O}}_{\text{2}}}\) in step 1 in terms of electrons.
State the change in oxidation number for manganese in step 2.
0.0002 moles of \({{\text{I}}^ – }\) were formed in step 3. Calculate the amount, in moles, of oxygen, \({{\text{O}}_{\text{2}}}\), dissolved in water.
▶️Answer/Explanation
Markscheme
amount of oxygen needed to decompose organic matter;
in a specified time/five days / at a specified temp/ 20 °C;
Second mark can only be awarded if reasonable attempt made to define BOD.
gained electrons;
+4 to +2 / decrease by 2;
\(0.00005/5 \times {10^{ – 5}}\) (moles);
Examiners report
In part (a) the term biochemical oxygen demand (BOD) was not well known. Very few candidates could explain that it is related to the level of organic waste in the water measured at a specific temperature for a specific time period.
Many candidates understood that oxygen gained electrons.
Many candidates understood that the oxidation number of manganese dropped from +4 to +2.
Many candidates understood that oxygen gained electrons in (c) (i) and that the oxidation number of manganese dropped from +4 to +2 in (ii). However, they struggled to calculate the moles of dissolved oxygen.
Question
In a class experiment, students were asked to determine the value of x in the formula of a hydrated salt, BaCl2・xH2O. They followed these instructions:
- Measure the mass of an empty crucible and lid.
- Add approximately 2 g sample of hydrated barium chloride to the crucible and record the mass.
- Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
- After cooling, reweigh the crucible, lid and contents.
- Repeat steps 3 and 4.
Their results in three trials were as follows:

State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for x in the hydrated salt.
List two assumptions made in this experiment.
▶️Answer/Explanation
Markscheme
repeat steps 3 and 4
OR
repeat step 5
OR
conduct a third heating
OR
«re»heat AND «re»weigh
water still present
OR
need two consistent readings
OR
heat to constant mass
Accept “ensure even/strong heating” for M1.
Do not accept “cleaning/washing the crucible”.
soot/carbon deposited
OR
incomplete combustion
OR
air hole of Bunsen burner closed/not fully open
Accept “using a yellow «Bunsen burner» flame” for M1.
«value of x» lower
Only award M2 if M1 correct.
all mass loss is due to water loss
all the water «of crystallization» is lost
crucible does not absorb/lose water
crystal/BaCl2 does not decompose/hydrolyse/oxidize/react with oxygen/air «when heated»
Accept “no loss of crystals/BaCl2 occurs”, “no impurities in the «weighed hydrated» salt”, “reaction goes to completion”, “heat was consistent/strong”, “crystal/BaCl2 does not absorb water during cooling”, “balance has been calibrated” or “crucible was clean at the start”.
Do not accept ”heat loss to surroundings” or “no carbon deposited on crucible”.
Reference to defects in apparatus not accepted.
Do not penalize if BaCl2.xH2O is used for BaCl2.
Question
In a class experiment, students were asked to determine the value of x in the formula of a hydrated salt, BaCl2・xH2O. They followed these instructions:
- Measure the mass of an empty crucible and lid.
- Add approximately 2 g sample of hydrated barium chloride to the crucible and record the mass.
- Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
- After cooling, reweigh the crucible, lid and contents.
- Repeat steps 3 and 4.
Their results in three trials were as follows:

State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for x in the hydrated salt.
List two assumptions made in this experiment.
▶️Answer/Explanation
Markscheme
repeat steps 3 and 4
OR
repeat step 5
OR
conduct a third heating
OR
«re»heat AND «re»weigh
water still present
OR
need two consistent readings
OR
heat to constant mass
Accept “ensure even/strong heating” for M1.
Do not accept “cleaning/washing the crucible”.
soot/carbon deposited
OR
incomplete combustion
OR
air hole of Bunsen burner closed/not fully open
Accept “using a yellow «Bunsen burner» flame” for M1.
«value of x» lower
Only award M2 if M1 correct.
all mass loss is due to water loss
all the water «of crystallization» is lost
crucible does not absorb/lose water
crystal/BaCl2 does not decompose/hydrolyse/oxidize/react with oxygen/air «when heated»
Accept “no loss of crystals/BaCl2 occurs”, “no impurities in the «weighed hydrated» salt”, “reaction goes to completion”, “heat was consistent/strong”, “crystal/BaCl2 does not absorb water during cooling”, “balance has been calibrated” or “crucible was clean at the start”.
Do not accept ”heat loss to surroundings” or “no carbon deposited on crucible”.
Reference to defects in apparatus not accepted.
Do not penalize if BaCl2.xH2O is used for BaCl2.
Question
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
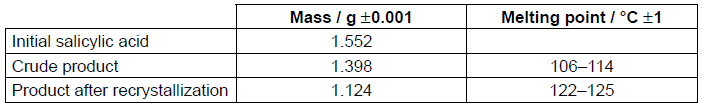
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
▶️Answer/Explanation
Markscheme
ALTERNATIVE 1:
«theoretical yield = \(\frac{{1.552\,{\text{g}}}}{{138.13\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\) × 180.17 g mol−1 =» 2.024 «g»
«experimental yield = \(\frac{{1.124{\rm{g}}}}{{2.024{\rm{g}}}}\) × 100 =» 55.53 «%»
ALTERNATIVE 2:
«\(\frac{{1.552\,{\text{g}}}}{{138.13\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)»= 0.01124 «mol salicylic acid/aspirin theoretical» AND
«\(\frac{{1.124\,{\text{g}}}}{{180.17\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)»= 0.006239 «mol aspirin experimental»
«experimental yield = \(\frac{{0.006239{\rm{mol}}}}{{0.01124{\rm{mol}}}}\) x 100 =» 55.51 «%»
Accept answers in the range 55.4 % to 55.7 %.
Award [2] for correct final answer.
low temperature gives greater difference between solubility of aspirin and impurities
OR
«product» crystallizes out from cold solution/«ice-cold water/lower temperature» speeds up crystallization process
OR
aspirin/product has low solubility «in water» at low temperatures
[N/A]
intercepts pain stimulus at source/acts at site of pain
OR
interferes with production of pain sensitizing substances/prostaglandins «at site of pain»
Examiners report
[N/A]
[N/A]
recrystallized melting point is higher
OR
recrystallized melting point is closer to pure substance/literature value
smaller range of values
Question
Sodium chloride, NaCl, can be spread on icy roads to lower the freezing point of water.
The diagram shows the effects of temperature and percentage by mass of NaCl on the composition of a mixture of NaCl and H2O.
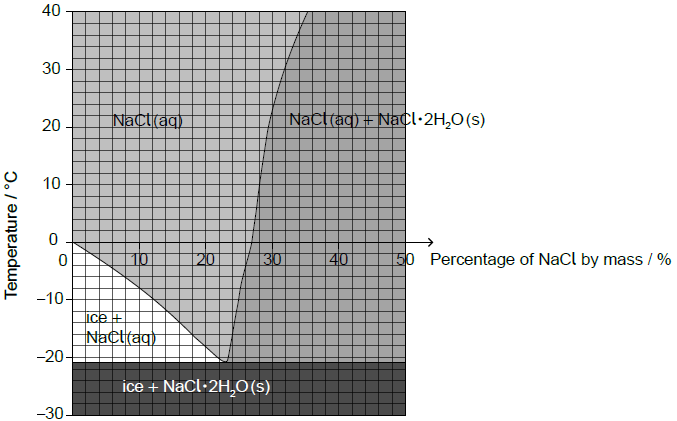
Estimate the lowest freezing point of water that can be reached by adding sodium chloride.
Estimate the percentage by mass of NaCl dissolved in a saturated sodium chloride solution at +10 ºC.
Calculate the percentage of water by mass in the NaCl•2H2O crystals. Use the data from section 6 of the data booklet and give your answer to two decimal places.
Suggest a concern about spreading sodium chloride on roads.
▶️Answer/Explanation
Markscheme
–21 «ºC»
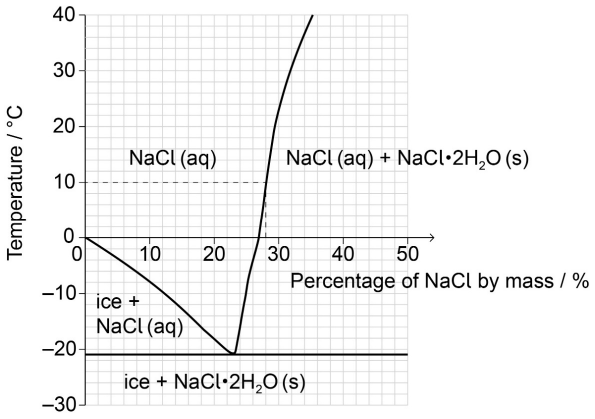
28 «%»
Accept any specific answer in the range 27 to 29 «%».
Mr = 94.48
«\(2\frac{{\left( {1.01 \times 2 + 16.00} \right)}}{{94.48}} \times 100 = \)» 38.15 «%»
Award M2 only if answer is to 2 decimal places.
Award [2] for correct final answer.
Award [1 max] for 38.10 %.
rust/corrosion «of cars and bridges»
OR
waste of important raw material
OR
soil/water salination/pollution «from run off»
OR
erosion of/damage to the road surface
OR
specific example of damage to the ecosystem
OR
«outdoor» temperatures may go below effective levels for NaCl «to lower freezing point» so NaCl could be wasted
OR
roads can refreeze causing hazards
Do not accept “tyre damage”.
Do not accept “economic issues” OR “environmental issues” unless specified (eg accept “increase in costs for local councils road budgets” but not “cost” alone).
Do not accept “makes roads more slippery”.
Question
Consider the following lipid and carbohydrate.
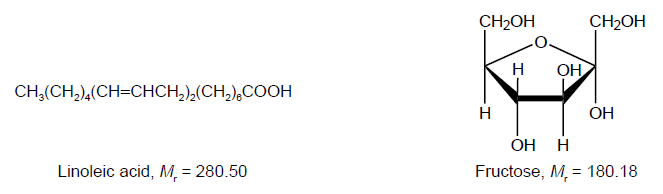
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
▶️Answer/Explanation
Markscheme
C9H16O
ratio of oxygen to carbon in linoleic acid lower
OR
linoleic acid less oxidized
OR
linoleic acid more reduced
Accept “«average» oxidation state of carbon in linoleic acid is lower”.
«electrophilic» addition/AE
OR
oxidation–reduction/redox
«\(\frac{{1.24\,{\text{g}}}}{{280.50\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\) =» 0.00442 «mol»
0.00884 mol of C=C
OR
ratio of linoleic acid : iodine = 1:2
«volume of I2 solution = \(\frac{{0.00884\,{\text{mol}}}}{{0.300\,{\text{mol}}\,{\text{d}}{{\text{m}}^{ – 3}}}}\) =» 0.0295 «dm3» / 29.5 «cm3»
Award [3] for correct final answer.
Any two of:
increases «ratio of» HDL «to LDL» cholesterol
OR
decreases LDL cholesterol «level»
removes plaque from/unblocks arteries
OR
decreases risk of heart disease
decreases risk of stroke «in the brain»
Accept “essential fatty acid”.
Do not accept “bad cholesterol” for “LDL cholesterol” OR “good cholesterol” for “HDL cholesterol”.
Do not accept general answers such as “source of energy” OR “forms triglycerides” OR “regulates permeability of cell membranes” etc.
[Max 2 Marks]
Question
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
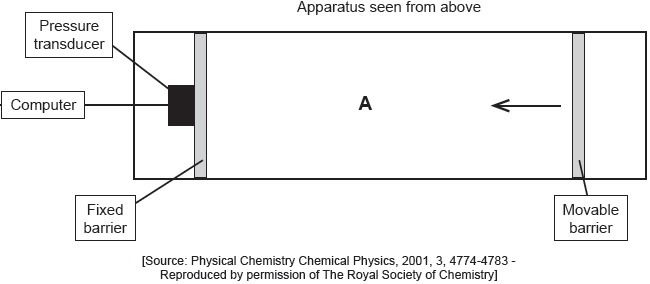
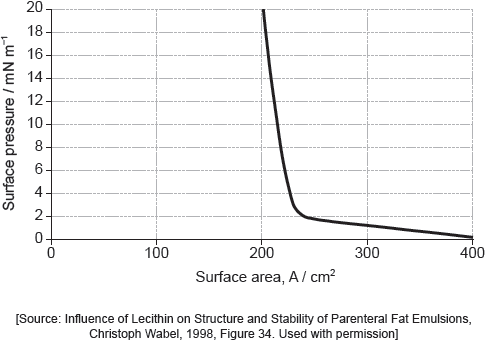
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
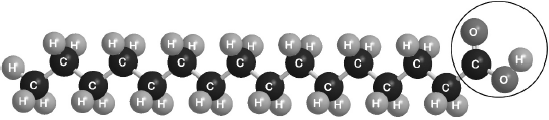
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.

The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
▶️Answer/Explanation
Markscheme
Must cut CH2–CO bond AND enclose all of the –COOH group.
[1 mark]
Any two of:
–COOH/CO/OH/carboxylate/carboxyl/hydroxyl/hydroxy group forms hydrogen bonds/H-bonds to water
London/dispersion/instantaneous induced dipole-induced dipole forces occur between hydrocarbon chains
hydrocarbon chain cannot form hydrogen bonds/H-bonds to water
strong hydrogen bonds/H-bonds between water molecules exclude hydrocarbon chains «from the body of the water»
Accept “hydrophilic part/group forms hydrogen bonds/H-bonds to water”.
Accept “hydrophobic section” instead of “hydrocarbon chain”.
Award [1 max] for answers based on “the –COOH group being polar AND the hydrocarbon chain being non-polar”.
[2 marks]
Above about 240 cm2:
greater collision frequency/collisions per second between «palmitic acid» molecules and the barrier «as area reduced»
At less than about 240 cm2:
molecules completely cover the surface
OR
there is no space between molecules
OR
force from movable barrier transmitted directly through the molecules to the fixed barrier
OR
«palmitic acid» molecules are pushed up/down/out of layer
For both M1 and M2 accept “particles” for “molecules”.
For M1 accept “space/area between molecules reduced” OR “molecules moving closer together”.
[2 marks]
amount of acid = «5.0 × 10–5 dm3 × 0.0034 mol dm–3» = 1.7 × 10–7 «mol»
number of molecules = «1.7 × 10–7 mol × 6.02 × 1023 mol–1 =» 1.0 × 1017
Award [2] for correct final answer.
Award [1] for “1.0 × 1020”.
[2 marks]
«area = \(\frac{{240{\text{ c}}{{\text{m}}^2}}}{{1.0 \times {{10}^{17}}}}\) » 2.4 × 10–15 «cm2»
[1 mark]

