Question
(a) State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane. [1]
(ii) Predict, giving a reason, whether ethane or chloroethane is more reactive. [1]
(iii) Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.[3]
(iv) Ethoxyethane (diethyl ether) can be used as a solvent for this conversion. Draw the structural formula of ethoxyethane. [1]
(v) Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet. [3]
(b) CCl2F2 is a common chlorofluorocarbon, CFC.
(i) Calculate the percentage by mass of chlorine in CCl2F2. [2]
(ii) Comment on how international cooperation has contributed to the lowering of CFC emissions responsible for ozone depletion. [1]
▶️Answer/Explanation
Ans
a i «free radical» substitution/SR Do not accept electrophilic ornucleophilic substitution.
a ii
chloroethane AND C–Cl bond is weaker/324 kJmol−1 than C–H bond/414 kJmol−1
OR
chloroethane AND contains a polar bond ✔
a iii
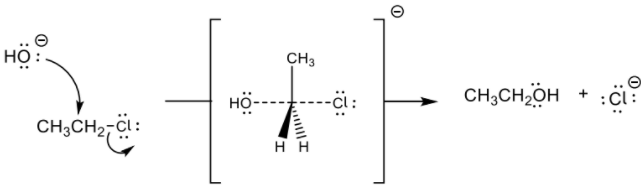
curly arrow going from lone pair/negative charge on O in −OH to C ✔
curly arrow showing Cl leaving ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
a iv 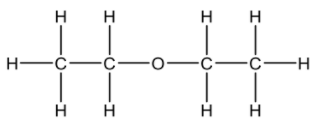 / CH3CH2OCH2CH3
/ CH3CH2OCH2CH3
a v
2 «signals» ✔
0.9−1.0 AND triplet ✔
3.3−3.7 AND quartet ✔
b i
«M (CCl2F2) =» 120.91 «g mol−1»
b ii
Any of:
research «collaboration» for alternative technologies «to replace CFCs»
OR
technologies «developed»/data could be shared
OR
political pressure/Montreal Protocol/governments passing legislations ✔
Question
(a) Write the equation and name the organic product when ethanol reacts with methanoic acid. [2]
Equation:
Product name:
(b) Oxidation of ethanol with potassium dichromate, K2Cr2O7, can form two different organic products. Determine the names of the organic products and the methods used to isolate them. [2]
▶️Answer/Explanation
Ans
a
Equation: CH3CH2OH + HCOOH ![]() HCOOCH2CH3 + H2O Product name: ethyl methanoate ✓
HCOOCH2CH3 + H2O Product name: ethyl methanoate ✓
Accept equation without equilibrium arrows.
Accept equation with molecular formulas (C2H6O + CH2O2 ![]() C3H6O2 + H2O) only if product name is correct.
C3H6O2 + H2O) only if product name is correct.
b
ethanal AND distillation ethanoic acid AND reflux «followed by distillation» ✓
Award [1 max] for both products OR both methods.
Question
Organic chemistry can be used to synthesize a variety of products.
(a) Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions. [2]
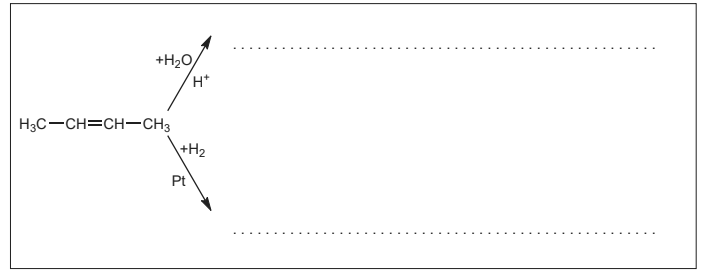
▶️Answer/Explanation
Ans
a
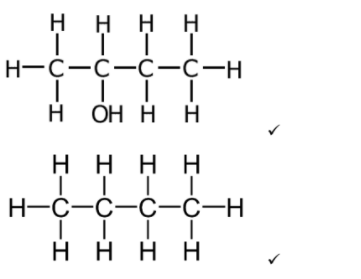
Penalize missing hydrogens in displayed structural formulas once only.
Accept condensed structural formulas: CH3CH(OH)CH2CH3 /CH3CH2CH2CH3 or skeletal structures.
Question
Chloroethene, C2H3Cl, is an important organic compound used to manufacture the polymer poly(chloroethene).
Draw the Lewis structure for chloroethene and predict the H–C–Cl bond angle.
Draw a section of poly(chloroethene) containing six carbon atoms.
Outline why the polymerization of alkenes is of economic importance and why the disposal of plastics is a problem.
Chloroethene can be converted to ethanol in two steps. For each step deduce an overall equation for the reaction taking place.
Step 1:
Step 2:
State the reagents and conditions necessary to prepare ethanoic acid from ethanol in the laboratory.
State an equation, including state symbols, for the reaction of ethanoic acid with water. Identify a Brønsted-Lowry acid in the equation and its conjugate base.
▶️Answer/Explanation
Markscheme
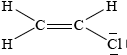 ;
;
Accept lines, dots or crosses for electron pairs.
Lone pairs required on chlorine.
(approximately) 120°;
Accept any bond angle in the range 113–120°.
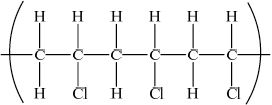 ;
;
Brackets not required for mark.
Continuation bonds from each carbon are required.
Cl atoms can be above or below carbon spine or alternating above and below.
plastics are cheap/versatile/a large industry / plastics have many uses / OWTTE;
plastics are not biodegradeable / plastics take up large amounts of space in landfill / pollution caused by burning of plastics / OWTTE;
Do not accept plastics cause litter.
Allow plastics don’t decompose quickly / OWTTE.
(i) Step 1:
\({\text{C}}{{\text{H}}_2}{\text{CHCl}} + {{\text{H}}_2} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{Cl}}\);
Step 2:
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{Cl}} + {\text{O}}{{\text{H}}^ – } \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{C}}{{\text{l}}^ – }\);
Allow NaOH or NaCl etc. instead of OH– and Cl–.
Allow abbreviated formulas C2H3Cl, C2H5Cl, C2H5OH.
\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\)/\({{\text{H}}^ + }\)/acidified and \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{_{\text{7}}}^{2 – }\)/(potassium/sodium) dichromate;
Accept suitable oxidizing agents (e.g. KMnO4 etc.) but only with acid.
Ignore missing or incorrect oxidation states in reagents.
(heat under) reflux;
Second mark can be scored even if reagent is incorrect.
\({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}\)
OR
\({\text{C}}{{\text{H}}_3}{\text{COOH(l)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}\)
OR
\({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\)
correct equation;
state symbols and \( \rightleftharpoons \);
BL acid is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and cb is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ – }\) / BL acid is \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and cb is \({{\text{H}}_{\text{2}}}{\text{O}}\);
Examiners report
The main G2 comments on this question related to the inclusion of organic chemistry in Section A. It should be noted that ANY Topic can be asked in Section A of P2, and there is no set-formula in relation to question setting. Organic chemistry is an integral part of the IB SL Chemistry programme, and is covered in Topic 10 of the guide (12 hours in total). Hence, candidates should be adequately prepared for questions on this topic, even in Section A. In 3(a), the Lewis structure of chlorethene was generally drawn correctly, though the weaker candidates often omitted the lone pairs on the chlorine. The bond angle was usually predicted, although right angles and 109.5° were often given. Even some of the better candidates explained their choice of bond angle, based on the fact that the double bond occupies more space causing the HCCl bond angle to drop less than 120°.
Many candidates gave double bonds and some forgot to include continuation bonds.
The Aim 8 question in part (iii) was very well answered this session. Almost all candidates scored the disposal problem of plastics mark and many achieved the economics importance mark also.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. (i) was poorly done. Candidates who managed a correct reaction for the first step often used water instead of hydroxide ion for the second step.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. In (ii), candidates who mentioned dichromate(VI) or permanganate(VIII) often omitted the acid. In addition, reflux was often missing.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. In (iii), very few candidates scored all three marks here, even though the question itself was easy. The equation was often correct, but the equilibrium arrow was rarely given. Some candidates did not know the formula for ethanoic acid which was surprising.
