Question
Stearic acid $\left(M_{\mathrm{r}}=284.47\right)$ and oleic acid $\left(M_{\mathrm{r}}=282.46\right)$ have the same number of carbon atoms. The structures of both lipids are shown in section 34 of the data booklet.
a. The iodine number is the number of grams of iodine which reacts with $100 \mathrm{~g}$ of fat. Calculate the iodine number of oleic acid.
[1]
b. State one impact on health of the increase in LDL cholesterol concentration in blood.
[1]
c. Explain why stearic acid has a higher melting point than oleic acid.
[2]
d(i)State one similarity and one difference in composition between phospholipids and triglycerides.
[2]
Similarity:
Difference:
d(ii)dentify a reagent that hydrolyses triglycerides.
[1]
▶️Answer/Explanation
Markscheme
a. «one $\mathrm{C}=\mathrm{C}$ bond»
«1 mole iodine : 1 mole oleic acid»
« $\frac{100 \times 253.80}{282.46}=$ » 89.85 «g of $\mathrm{I}_2$ “
NOTE: Accept “90 “g of $\mathrm{I}_2$ “).
b. atherosclerosis/cholesterol deposition «in artery walls»/increases risk of heart attack/stroke/cardiovascular disease/CHD
NOTE: Accept “arteries become blocked/walls become thicker”, “increases blood pressure”, oR “blood clots”.
Do not accept “high cholesterol” OR “obesity”
c. no kinks in chain/more regular structure
OR
straight chain
OR
no $\mathrm{C}=\mathrm{C} /$ carbon to carbon double bonds
OR
saturated
OR
chains pack more closely together
NOTE: Accept “greater surface area/electron density” for M1.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
NOTE: Accept “stronger intermolecular/van der Waals’/vdW forces” for M2.
d(i)Similarity:
«derived from» propane-1,2,3-triol/glycerol/glycerin/glycerine
OR
«derived from» at least two fatty acids
OR
contains ester linkages
OR
long carbon chains
NOTE: Do not accept “two fatty acids as both a similarity and a difference”.
Do not accept just “hydrocarbon/carbon chains”.
Difference:
phospholipids contain two fatty acids «condensed onto glycerol» $A N D$ triglycerides three
OR
phospholipids contain phosphate/phosphato «group»/residue of phosphoric acid $A N D$ triglycerides do not
NOTE: Accept “phospholipids contain phosphorus AND triglycerides do not”.
Accept “phospholipids are amphiphilic AND triglycerides are not” OR “phospholipids have hydrophobic tails and hydrophilic heads AND triglycerides do not”.
d(ii)sconcentrated» $\mathrm{NaOH}(\mathrm{aq}) /$ sodium hydroxide
OR
«concentrated» $\mathrm{HCl}(\mathrm{aq}) /$ hydrochloric acid
OR
enzymes/lipases
NOTE: Accept other strong acids or bases.
Question
Ethanol is a biofuel that can be mixed with gasoline.
a. Write the equation for the complete combustion of ethanol.
b. Outline the evidence that relates global warming to increasing concentrations of greenhouse gases in the atmosphere.
c. Explain, including a suitable equation, why biofuels are considered to be carbon neutral.
d. State the type of reaction that occurs when ethanol reacts with vegetable oil to form biodiesel.
▶️Answer/Explanation
Markscheme
a. $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{l})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
(I)
b. Any two of:
«showing strong» correlation between «atmospheric» $\mathrm{CO}_2$ concentration/greenhouse gas concentration and average «global/surface/ocean» temperature
lab evidence that greenhouse gases $/ \mathrm{CO}_2$ absorb(s) infrared radiation «advanced» computer modelling
ice core data
tree ring data
ocean sediments / coral reefs / sedimentary rocks data
NOTE: Do not accept “global warming” for “average temperature”.
Do not accept “traps/reflects heat” OR “thermal energy”.
Evidence must be outlined and connected to data.
Accept references to other valid greenhouse gases other than carbon dioxide/ $\mathrm{CO}_2$, such as methane/ $\mathrm{CH}_4$ or nitrous oxide/ $\mathrm{N}_2 \mathrm{O}$.
c. biofuel raw material/sugar/glucose formed by photosynthesis
OR
biofuel raw material/sugar/glucose uses up carbon dioxide during its formation
OR
biofuel from capturing gases due to decaying organic matter formed from photosynthesis
$6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2(\mathrm{~g})$
NOTE: Accept arguments based on material coming from plant sources consuming carbon dioxide/carbon for M1.
d. transesterification
OR
«nucleophilic» substitution $/ \mathrm{S}_{\mathrm{N}}$
Question
Ethanol is a depressant that is widely consumed in many societies. When consumed excessively it has a major impact on families and society as a whole. Other depressants such as diazepam (Valium®) may be prescribed by a doctor.
One problem associated with ethanol consumption is an increased risk of traffic accidents. Police in many countries use a breathalyser to test drivers. The breathalyser contains potassium dichromate(VI).
Describe the colour change of potassium dichromate(VI) when it reacts with ethanol.
State with a reason whether chromium in potassium dichromate(VI) is oxidised or reduced by ethanol.
▶️Answer/Explanation
Markscheme
orange to green;
reduced because oxidation number of Cr decreases / Cr gains electrons;
Explanation needed for mark.
Examiners report
[N/A]
Candidates frequently confused oxidation and reduction or failed to provide a reason as to whether the chromium was oxidised or reduced by ethanol. This highlighted, again, the need for candidates to answer all parts of the question.
Question
Alkenes can undergo electrophilic addition reactions with bromine and with hydrogen bromide.
Name the product formed when but-2-ene reacts with
Explain how a bromine molecule is able to act as an electrophile.
(i) bromine.
(ii) hydrogen bromide.
When but-1-ene reacts with hydrogen bromide, two possible organic products could be formed but in practice only one organic product is obtained in high yield. Explain the mechanism for this reaction using curly arrows to represent the movement of electron pairs and explain clearly why only one organic product is formed.
▶️Answer/Explanation
Markscheme
as the bromine approaches the alkene an induced dipole is formed / OWTTE;
(i) 2,3-dibromobutane;
(ii) 2-bromobutane;
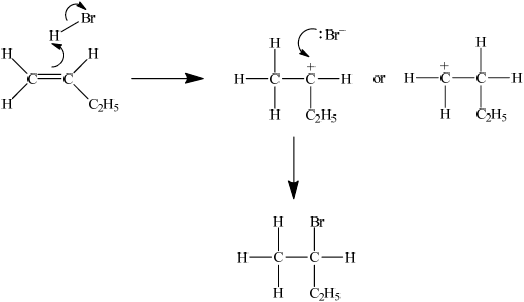
showing curly arrow from double bond to H (in H–Br) and curly arrow from bond in H–Br to Br;
showing the curly arrow from the lone pair/negative charge on Br– to the secondary carbocation and 2-bromobutane as correct product;
stating that the secondary carbocation will be formed in preference to the primary carbocation;
the two positive/electron releasing inductive effects due to the two R– groups on the secondary carbocation make it more stable;
Examiners report
In part (a) candidates rarely explained the induced dipole in the bromine molecule which allows it to act as an electrophile.
Part (b) was answered more effectively with many candidates correctly naming products formed from but-2-ene, although several candidates omitted ‘di’ from 2,3-dibromobutane and thus lost the mark.
The poor use of curly arrows was again evident in part (c) although some candidates clearly explained why only one organic product is formed when but-1-ene reacts with hydrogen bromide.
Question
Hydrolysis of aliphatic and aromatic halides occurs under different conditions.
State an equation, using structural formulas, to show the reaction of 1-chloro-2-(chloromethyl) benzene with excess sodium hydroxide at room temperature.
▶️Answer/Explanation
Markscheme
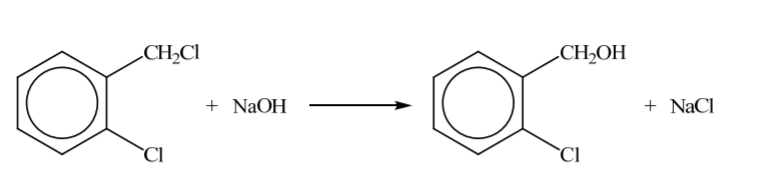
correct formulas of the reactants and the inorganic product;
correct formula of the organic product;
Examiners report
The difference in the ease with which aliphatic and aromatic halides hydrolyse was poorly understood.
Question
Fats and oils have some similarities and some differences in their chemical structures.
State two major differences in their structures.
Describe how an oil can be converted into a fat.
Discuss two advantages and two disadvantages of converting oils into fats.
▶️Answer/Explanation
Markscheme
oils contain at least one C=C/carbon to carbon double bond;
oils have fewer carbon atoms in the hydrocarbon chains / OWTTE;
hydrogenation / react with hydrogen (gas);
heat/140−225 °C and metal catalyst/Ni/Zn/Cu/pressure;
Advantages: [2 max]
increases melting points / changes oil to a semi-solid/solid;
decreases rate of oxidation;
increases hardness;
controls feel/plasticity/stiffness;
Disadvantages: [2 max]
the more saturated the less good for the heart / OWTTE;
trans-fatty acids can be formed (through partial hydrogenation);
trans-fatty acids are difficult to metabolize / increase LDL levels / low quality energy source / accumulate in fatty tissue / are difficult to digest/excrete (from the body);
Examiners report
Most candidates compared structural features of fats and oils, but many failed to score as they missed the required specificity of carbon to carbon double bond in (a). A significant number of candidates compared melting points which was not part of the question and very few were able to state the difference in the length of hydrocarbon chains.
Many candidates gave detailed descriptions of the process to score both marks in part (b), but some failed to score the second mark by omitting the need of a catalyst/pressure and/or heat.
Many candidates were able to correctly suggest two advantages but failed to correctly state two disadvantages in part (c). Very often marks were lost as result of poor use of subject specific terms.
Question
The cumene process is used for the production of both propanone and phenol. The overall reaction is shown in the equation below.
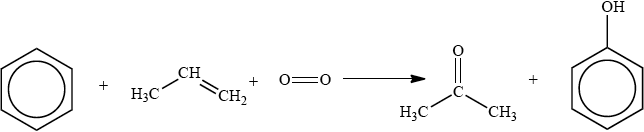
This process is important in the polymer industry. Propanone can be converted into methyl methacrylate, the monomer used to make Perspex®, and phenol is used in phenol-methanal resins, which are important thermosetting plastics.
State and explain how the presence of a halogen substituent might affect the acidity of carboxylic acids.
Propanone could also be formed from propene by reaction with steam over an acidic catalyst, followed by oxidation of the product.
The reaction of propene with water can yield two possible products. Explain, in terms of the stability of the intermediate carbocations, why one is formed in much greater quantities than the other.
▶️Answer/Explanation
Markscheme
halogens make them more acidic;
halogens are electron withdrawing;
Accept halogens (can be) electronegative.
reduces charge on/stabilizes anion formed / weakens O–H bond / makes it easier to lose \({{\text{H}}^ + }\) ion;
Accept decreases pKa.
Accept causes anion to be weaker base.
one product involves a primary carbocation and other a secondary carbocation;
secondary carbocation is more stable (than the primary carbocation, and hence this produces the major product);
alkyl groups reduce charge on carbon atom (through an inductive effect);
Positive inductive effect of alkyl groups alone not enough for M3.
Examiners report
(a) (i) was well done by the better candidates only, but most candidates only scored one mark in (ii) and no marks in (iii).
(d) was very poorly answered. Some knew that there was an inductive effect but did not understand what this meant, namely that through the positive inductive effect the alkyl groups reduce the charge on the carbon atom.
Question
In a class experiment, students were asked to determine the value of x in the formula of a hydrated salt, BaCl2・xH2O. They followed these instructions:
- Measure the mass of an empty crucible and lid.
- Add approximately 2 g sample of hydrated barium chloride to the crucible and record the mass.
- Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
- After cooling, reweigh the crucible, lid and contents.
- Repeat steps 3 and 4.
Their results in three trials were as follows:
State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for x in the hydrated salt.
List two assumptions made in this experiment.
▶️Answer/Explanation
Markscheme
repeat steps 3 and 4
OR
repeat step 5
OR
conduct a third heating
OR
«re»heat AND «re»weigh
water still present
OR
need two consistent readings
OR
heat to constant mass
Accept “ensure even/strong heating” for M1.
Do not accept “cleaning/washing the crucible”.
soot/carbon deposited
OR
incomplete combustion
OR
air hole of Bunsen burner closed/not fully open
Accept “using a yellow «Bunsen burner» flame” for M1.
«value of x» lower
Only award M2 if M1 correct.
all mass loss is due to water loss
all the water «of crystallization» is lost
crucible does not absorb/lose water
crystal/BaCl2 does not decompose/hydrolyse/oxidize/react with oxygen/air «when heated»
Accept “no loss of crystals/BaCl2 occurs”, “no impurities in the «weighed hydrated» salt”, “reaction goes to completion”, “heat was consistent/strong”, “crystal/BaCl2 does not absorb water during cooling”, “balance has been calibrated” or “crucible was clean at the start”.
Do not accept ”heat loss to surroundings” or “no carbon deposited on crucible”.
Reference to defects in apparatus not accepted.
Do not penalize if BaCl2.xH2O is used for BaCl2.
Question
Oseltamivir (Tamiflu) and zanamivir (Relenza) are both used as antivirals to help prevent the spread of the flu virus, but are administered by different methods.
Zanamivir must be taken by inhalation, not orally. Deduce what this suggests about the bioavailability of zanamivir if taken orally.
Oseltamivir does not possess the carboxyl group needed for activity until it is chemically changed in the body. Deduce the name of the functional group in oseltamivir which changes into a carboxyl group in the body. Use section 37 of the data booklet.
The synthesis of oseltamivir is dependent on a supply of the precursor shikimic acid, which is available only in low yield from certain plants, notably Chinese star anise. State one alternative green chemistry source of shikimic acid.
▶️Answer/Explanation
Markscheme
«oral bioavailability is» low
OR
drug is broken down/pH too low/unable to be absorbed from gut
OR
only a small proportion of the drug «taken by mouth» reaches the target organ
ethoxycarbonyl/carbonyl attached to oxygen
Accept “ester”.
Any one of:
fermentation
OR
microbial production
genetically engineered bacteria/E.coli
sweetgum «seeds/leaves/bark»
OR
pine/fir/spruce tree «needles»
OR
Ginkgo biloba
Accept other specific examples of more plentiful plant sources.
Question
Consider the following lipid and carbohydrate.
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
▶️Answer/Explanation
Markscheme
C9H16O
ratio of oxygen to carbon in linoleic acid lower
OR
linoleic acid less oxidized
OR
linoleic acid more reduced
Accept “«average» oxidation state of carbon in linoleic acid is lower”.
«electrophilic» addition/AE
OR
oxidation–reduction/redox
«\(\frac{{1.24\,{\text{g}}}}{{280.50\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\) =» 0.00442 «mol»
0.00884 mol of C=C
OR
ratio of linoleic acid : iodine = 1:2
«volume of I2 solution = \(\frac{{0.00884\,{\text{mol}}}}{{0.300\,{\text{mol}}\,{\text{d}}{{\text{m}}^{ – 3}}}}\) =» 0.0295 «dm3» / 29.5 «cm3»
Award [3] for correct final answer.
Any two of:
increases «ratio of» HDL «to LDL» cholesterol
OR
decreases LDL cholesterol «level»
removes plaque from/unblocks arteries
OR
decreases risk of heart disease
decreases risk of stroke «in the brain»
Accept “essential fatty acid”.
Do not accept “bad cholesterol” for “LDL cholesterol” OR “good cholesterol” for “HDL cholesterol”.
Do not accept general answers such as “source of energy” OR “forms triglycerides” OR “regulates permeability of cell membranes” etc.
[Max 2 Marks]

