Question
In order to determine the oil content of different types of potato crisps (chips), a student weighed $5.00 \mathrm{~g}$ of crushed crisps and mixed them with
20. $0 \mathrm{~cm}^3$ of non-polar solvent.
She assumed all the oil in the crisps dissolved in the solvent.
The student then filtered the mixture to remove any solids, and gently heated the solution on a hot plate to evaporate the solvent.
She measured the mass of the oil that remained from each type of crisps
a. Suggest why a non-polar solvent was needed.
b. State one reason why the mixture was not heated strongly.
c. Non-polar solvents can be toxic. Suggest a modification to the experiment which allows the evaporated solvent to be collected.
d. Suggest one source of error in the experiment, excluding faulty apparatus and human error, that would lead to the following: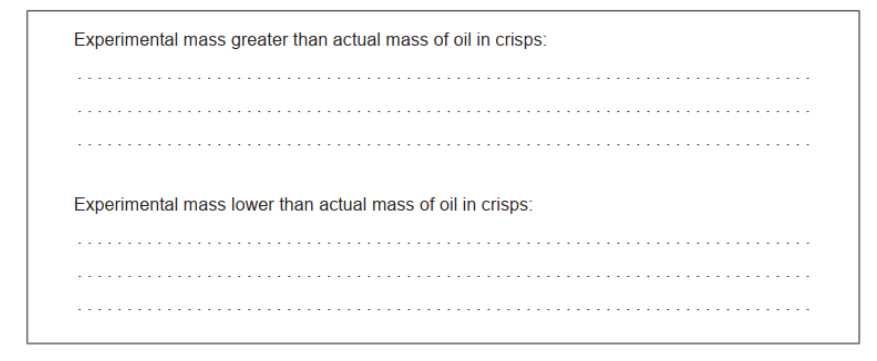
▶️Answer/Explanation
Markscheme
a. oil is non-polar «and dissolves best in non-polar solvents»
OR
oil does not dissolve in polar solvents
Do not accept “like dissolves like” only.
b. solvent/oil is flammable
OR
solvent/oil must be kept below its flash point
OR
oxidation/decomposition of oil
OR
mixture has a low boiling point
Accept “to prevent evaporation of oil”.
c. distillation «instead of evaporation»
Accept “pass vapour through a condenser and collect liquid”.
Do not accept “condensation” without experimental details.
d. Experimental mass greater than actual mass of oil in crisps: other substances «in the crisps» are soluble in the solvent
OR
not all the solvent evaporates
Experimental mass less than actual mass of oil in crisps:
not all oil dissolved/extracted
Accept “oil evaporated” OR “oil burned/decomposed” OR “oil absorbed by the filter” OR “assumption “all oil dissolved” was wrong” for M2.
Do not accept examples of human errors OR faulty apparatus.
Question
An investigation was carried out to determine the effect of chain length of the alcohol on the equilibrium constant, $K_{\mathrm{c}}$, for the reversible reaction:
$$
\mathrm{ROH}+\mathrm{CH}_3 \mathrm{COOH} \stackrel{\mathrm{H}^{+}(\mathrm{aq})}{\rightleftharpoons} \mathrm{CH}_3 \mathrm{COOR}+\mathrm{H}_2 \mathrm{O}
$$
The reactants, products and the catalyst form a homogeneous mixture.
Fixed volumes of each alcohol, the ethanoic acid and the sulfuric acid catalyst were placed in sealed conical flasks.
At equilibrium, the flasks were placed in an ice bath, and samples of each flask titrated with $\mathrm{NaOH}(\mathrm{aq})$ to determine the ethanoic acid concentration present in the equilibrium mixture.
The following processed results were obtained.
a.Identify the independent and dependent variables in this experiment.
b. The ice bath is used at equilibrium to slow down the forward and reverse reactions. Explain why adding a large amount of water to the reaction
[2] mixture would also slow down both reactions.
c. Suggest why the titration must be conducted quickly even though a low temperature is maintained.
d. An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with $\mathrm{NaOH}$ (aq). Outline why this experiment was necessary.
e. Calculate the percentage uncertainty and percentage error in the experimentally determined value of $K_{\mathrm{c}}$ for methanol.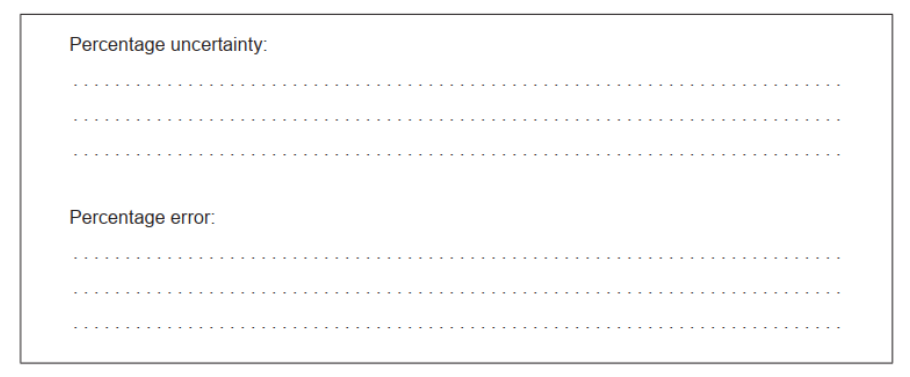
f. Comment on the magnitudes of random and systematic errors in this experiment using the answers in (e).
g. Suggest a risk of using sulfuric acid as the catalyst.
▶️Answer/Explanation
Markscheme
a. Independent variable:
chain length $O R$ number of carbon «atoms in alcohol»
AND
Dependent variable:
volume of $\mathrm{NaOH} O R K_c$ lequilibrium constant $O R$ equilibrium concentration $/ \mathrm{moles}$ of $\mathrm{CH}_3 \mathrm{COOH}$
b. dilution/lower concentrations
less frequent collisions «per unit volume»
Accept “lowers concentration of acid catalyst” for M1. M2 must refer to increase in activation energy or different pathway.
Do not accept responses referring to equilibrium.
c. equilibrium shifts to left
OR
more ethanoic acid is produced «as ethanoic acid is neutralized»
OR
prevents/slows down ester hydrolysis
Accept “prevents equilibrium shift” if described correctly without direction.
d. to determine volume/moles of $\mathrm{NaOH}$ used up by the catalyst/sulfuric acid «in the titration»
OR
to eliminate/reduce «systematic» error caused by acid catalyst
Do not accept “control” OR “standard” alone.
e. Percentage uncertainty:
$$
« \frac{0.4 \times 100}{6.5}=» 6 \ll \%
$$
Percentage error:
$$
« \frac{6.5-5.3}{5.3}=» 23 \ll \% »
$$
Award [1 max] if calculations are reversed OR if incorrect alcohol is used.
f. Any two:
large percentage error means large systematic error «in procedure» small percentage uncertainty means small random errors random errors smaller than systematic error
Award [2] for “both random and systematic errors are significant.”
g. corrosive/burns/irritant/strong oxidizing agent/carcinogenic OR disposal is an environmental issue OR causes other side reactions/dehydration/decomposition
Do not accept just “risk of accidents” OR “health risks” OR “hazardous”.
Question
Sodium chloride, $\mathrm{NaCl}$, can be spread on icy roads to lower the freezing point of water.
The diagram shows the effects of temperature and percentage by mass of $\mathrm{NaCl}$ on the composition of a mixture of $\mathrm{NaCl}$ and $\mathrm{H}_2 \mathrm{O}$.
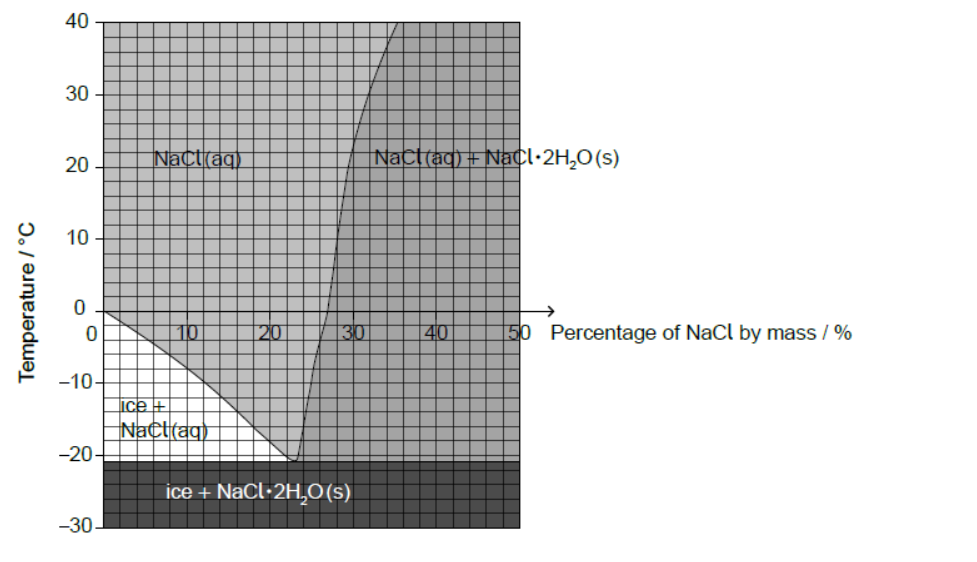
a. Estimate the lowest freezing point of water that can be reached by adding sodium chloride.
b. Estimate the percentage by mass of $\mathrm{NaCl}$ dissolved in a saturated sodium chloride solution at $+10^{\circ} \mathrm{C}$.
[1]
c. Calculate the percentage of water by mass in the $\mathrm{NaCl} \cdot 2 \mathrm{H}_2 \mathrm{O}$ crystals. Use the data from section 6 of the data booklet and give your answer to two decimal places.
d. Suggest a concern about spreading sodium chloride on roads.
▶️Answer/Explanation
Markscheme
a. -21 ” ${ }^{\circ} \mathrm{C} »$
b,
28 «\%»
Accept any specific answer in the range 27 to 29 «\%».
c. $M_{\mathrm{r}}=94.48$
$« 2 \frac{(1.01 \times 2+16.00)}{94.48} \times 100=338.15$ «\%»
Award M2 only if answer is to 2 decimal places.
Award [2] for correct final answer.
Award [1 max] for $38.10 \%$.
d. rust/corrosion «of cars and bridges»
OR
waste of important raw material
OR
soil/water salination/pollution «from run off»
OR
erosion of/damage to the road surface
OR
specific example of damage to the ecosystem
OR
«outdoor» temperatures may go below effective levels for $\mathrm{NaCl}$ «to lower freezing point» so $\mathrm{NaCl}$ could be wasted
$O R$
roads can refreeze causing hazards
Do not accept “tyre damage”.
Do not accept “economic issues” OR “environmental issues” unless specified (eg accept “increase in costs for local councils road budgets” but not “cost” alone).
Do not accept “makes roads more slippery”.
Question
Alloys containing at least $60 \%$ copper reduce the presence of bacteria on their surface. The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to $250.0 \mathrm{~cm}^3$ with water before analysis.
$$
\begin{aligned}
& \mathrm{Cu}(\mathrm{s})+4 \mathrm{HNO}_3(\mathrm{aq}) \rightarrow \mathrm{Cu}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{NO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
& 3 \mathrm{Zn}(\mathrm{s})+8 \mathrm{HNO}_3(\mathrm{aq}) \rightarrow 3 \mathrm{Zn}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{NO}(\mathrm{g})+4 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
&
\end{aligned}
$$
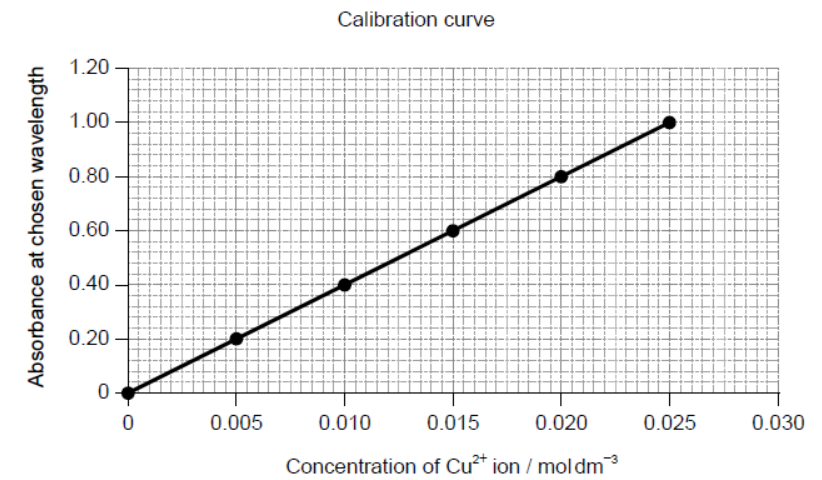
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
a. Outline why the initial reaction should be carried out under a fume hood.
b. Deduce the equation for the relationship between absorbance and concentration.
c. Outline how a solution of $0.0100 \mathrm{~mol} \mathrm{dm}^{-3}$ is obtained from a standard $1.000 \mathrm{~mol} \mathrm{dm}^{-3}$ copper(II) sulfate solution, including two essential pieces
[3] of glassware you would need.
d.i. The original piece of brass weighed $0.200 \mathrm{~g}$. The absorbance was 0.32 .
Calculate, showing your working, the percentage of copper by mass in the brass.
d.ii.Deduce the appropriate number of significant figures for your answer in (d)(i).
e.i. Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (d)(i), use $70 \%$ but this is not the correct answer.
e.ii.Suggest another property of brass that makes it suitable for door handles.
f.i. Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ (aq). Copper(I) iodide is a white solid.
$$
\begin{gathered}
4 \mathrm{I}^{-}(\mathrm{aq})+2 \mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cul}(\mathrm{s})+\mathrm{I}_2(\mathrm{aq}) \\
\mathrm{I}_2(\mathrm{aq})+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}(\mathrm{aq}) \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq})+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}(\mathrm{aq})
\end{gathered}
$$
Deduce the overall equation for the two reactions by combining the two equations.
f.ii. Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
▶️Answer/Explanation
Markscheme
a. $\mathrm{NO}_2 / \mathrm{NO} / \mathrm{NO}_{\mathrm{x}} / \mathrm{HNO}_3 /$ gas is poisonous/toxic/irritant
Accept formula or name.
Accept “HNO ${ }_3$ is corrosive” OR “poisonous/toxic gases produced”.
Accept “reaction is harmful/hazardous”.
b. Slope (gradient):
40
Equation:
absorbance $=40 \times$ concentration
OR
$y=40 x$
Accept any correct relationship for slope such as $\frac{1.00}{0.025}$.
Award [2] if equation in M2 is correct.
c. dilute $1.00 \mathrm{~cm}^3$ «of the standard solution with water» to $100 \mathrm{~cm}^3$
OR
dilute sample of standard solution «with water» 100 times
«graduated/volumetric» pipette/pipet
volumetric flask
Accept any 1: 100 ratio for $M 1$.
Accept “mix $1 \mathrm{~cm}^3$ of the standard solution with $99 \mathrm{~cm}^3$ of water” for M1.
Do not accept “add $100 \mathrm{~cm}^3$ of water to $1.00 \mathrm{~cm}^3$ of standard solution” for M1.
Accept “burette/buret” for M2.
Accept “graduated/measuring flask” for M3 but not “graduated/measuring cylinder” or “conical/Erlenmeyer flask”.
d.i. concentration of copper $=0.0080 \ll \mathrm{mol} \mathrm{dm^{-3 } »}$
mass of copper in $250.0 \mathrm{~cm}^3=\ll 0.0080 \mathrm{~mol} \mathrm{dm}^{-3} \times 0.2500 \mathrm{dm}^3 \times 63.55 \mathrm{~g} \mathrm{~mol}^{-1}=» 0.127$ «»
OR
mass of brass in $1 \mathrm{dm}^3=« 4 \times 0.200 \mathrm{~g}=» 0.800 \mathrm{~g} \mathrm{AND}$ [Cu2+] $=« 0.0080 \mathrm{~mol} \mathrm{dm}^{-3} \times 63.55 \mathrm{~g} \mathrm{~mol}^{-1}=» 0.5084 \mathrm{~g} \mathrm{dm}^{-3}$
«\% copper in this sample of brass $=\frac{0.127}{0.200} \times 100=» 64 « \% »$
OR
$« \%$ copper in this sample of brass $=\frac{0.5084}{0.800} \times 100=» 64 \ll \% »$
Accept annotation on graph for M1.
Award [3] for correct final answer.
Accept “65 «\%»”.
d.ii.wo
Do not apply ECF from 1(d)(i).
e.i. «since it is greater than $60 \%$ » it will reduce the presence of bacteria «on door handles»
e.iiresistant to corrosion/oxidation/rusting
OR
low friction surface «so ideal for connected moving components»
Accept “hard/durable”, “«high tensile» strength”, “unreactive”, “malleable” or any reference to the appearance/colour of brass (eg “gold-like”, “looks nice” etc.).
Do not accept irrelevant properties, such as “high melting/boiling point”, “non-magnetic”, “good heat/electrical conductor”, “low volatility”, etc. Do not accept “ductile”.
f.i. $2 \mathrm{I}^{-}(\mathrm{aq})+2 \mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}(\mathrm{aq}) \rightarrow 2 \mathrm{Cul}(\mathrm{s})+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}(\mathrm{aq})$
correct reactants and products
balanced equation
M2 can only be awarded if $M 1$ is correct.
f.ii. precipitate/copper(I) iodide/Cul makes colour change difficult to see
OR
release of $\mathrm{I}_2$ /iodine from starch- $\mathrm{I}_2$ complex is slow so titration must be done slowly
Question
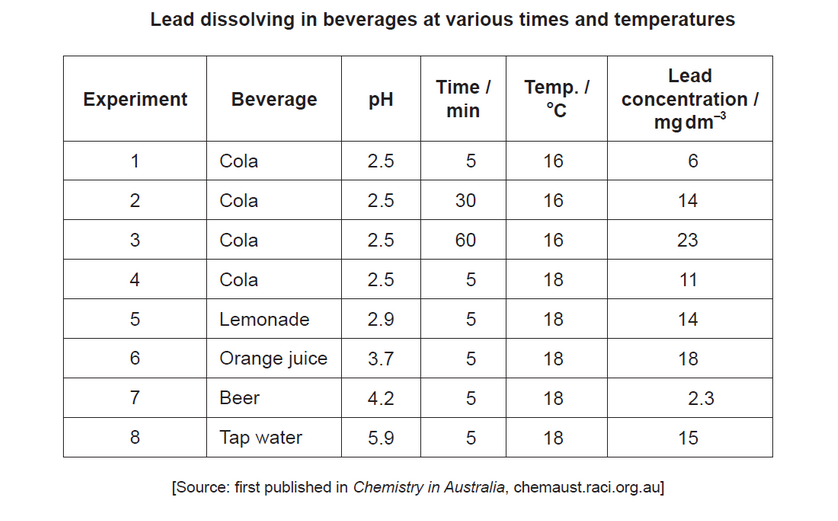
This question is about a mug made of a lead alloy.

The rate of lead dissolving in common beverages with various pH values was analysed.
Bromine and methanoic acid react in aqueous solution.
$$
\mathrm{Br}_2(\mathrm{aq})+\mathrm{HCOOH}(\mathrm{aq}) \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq})+2 \mathrm{H}^{+}(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g})
$$
The reaction was monitored by measuring the volume of carbon dioxide produced as time progressed.
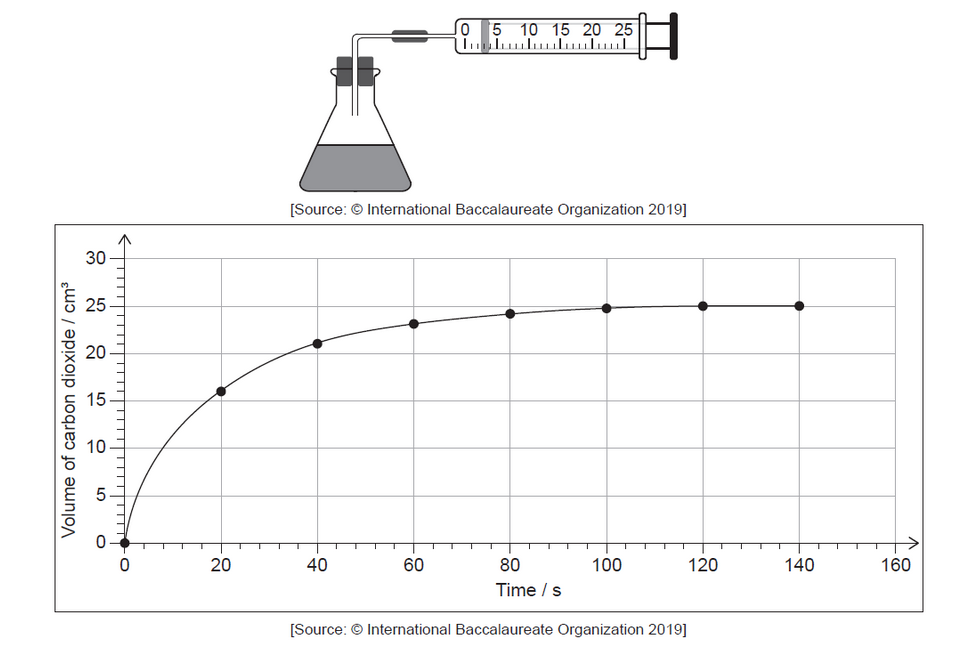
a. Determine from the graph the rate of reaction at $20 \mathrm{~s}$, in $\mathrm{cm}^3 \mathrm{~s}^{-1}$, showing your working.
b. Outline, with a reason, another property that could be monitored to measure the rate of this reaction.
b(iiExamine, giving a reason, whether the rate of lead dissolving increases with acidity at $18^{\circ} \mathrm{C}$.
c(i)Describe one systematic error associated with the use of the gas syringe, and how the error affects the calculated rate.
c(ii)dentify one error associated with the use of an accurate stopwatch.
▶️Answer/Explanation
Markscheme
a. tangent drawn to curve at $\mathrm{t}=20 \mathrm{~s}[\boldsymbol{V}]$
slope/gradient calculation $[\boldsymbol{C}]$
$0.35 « \mathrm{~cm}^3 \mathrm{~s}^{-1} »[$ [ ]
Note: Accept values in the range $0.32-0.42 ” « \mathrm{~cm}^3 \mathrm{~s}^{-1} \rrbracket$.
b. ALTERNATIVE 1
colour [ $\checkmark]$
$\mathrm{Br}_2 /$ reactant is coloured «Br (aq)/product is not» [ $\left.\boldsymbol{D}\right]$
Note: Do not accept “changes in temperature” or “number of bubbles”.
ALTERNATIVE 2
conductivity [ $/]$
greater/increased concentration of ions in products [ $U$ ]
ALTERNATIVE 3
mass/pressure $[\boldsymbol{V}]$
gas is evolved/produced [ $\boldsymbol{V}]$
Note: Do not accept “mass of products is less than mass of reactants”.
ALTERNATIVE 4
$\mathrm{pH}[\leftrightharpoons$
methanoic acid is weak $A N D \mathrm{HBr}$ is strong
OR
increase in $\left[\mathrm{H}^{+}\right][\boldsymbol{U}]$
b(ii)o AND experiment $7 /$ beer has lowest rate and intermediate acidity/pH
OR
no AND experiment 6/orange juice has fastest rate but lower acidity/higher $\mathrm{pH}$ than experiment $5 /$ lemonade
OR
no $A N D$ experiment 6/orange juice has highest rate and intermediate acidity/pH [ $\boldsymbol{\sim}]$
Note: Accept no AND any comparison, with experimental support, that concludes no pattern/increase with acidity.
eg: “rate of $\mathrm{Pb} / \mathrm{lead}$ dissolving generally decreases with acidity as tap water has highest rate (after orange juice) while lemonade (lower $\mathrm{pH}$ ) has lower rate”.
c(i)ALTERNATIVE 1
gas may leak/be lost/escape
OR
plunger may stick/friction «so pressure is greater than atmospheric pressure»
OR
syringe may be tilted «up» so plunger moves less «with gravity acting on plunger»
OR
$\mathrm{CO}_2$ dissolved in water $[\boldsymbol{V}]$
calculated rate lower
ALTERNATIVE 2
syringe may be tilted «down» so plunger moves more «with gravity acting on plunger» OR syringe is held in hand so gets warmer and gas expands [ $M$ ] calculated rate higher $[\boldsymbol{\sim}]$
Note: Calculated rate is lower or higher must be stated for $M 2$.
Do not accept “scale on syringe is inaccurate”, “errors in reading syringe”, or “bubbles in syringe”.
c(ii)uman reaction time/delay «starting/stopping the stopwatch» [ $M$ ]
Note: Do not accept “inaccurate stopwatch”.
Question
\text { A student investigated how the type of acid in acid deposition affects limestone, a building material mainly composed of calcium carbonate. }
The student monitored the mass of six similarly sized pieces of limestone. Three were placed in beakers containing $200.0 \mathrm{~cm}^3$ of $0.100 \mathrm{~mol} \mathrm{dm}^{-3}$ nitric acid, $\mathrm{HNO}_3(\mathrm{aq})$, and the other three in $200.0 \mathrm{~cm}^3$ of $0.100 \mathrm{~mol} \mathrm{dm}^{-3}$ sulfuric acid, $\mathrm{H}_2 \mathrm{SO}_4$ (aq).
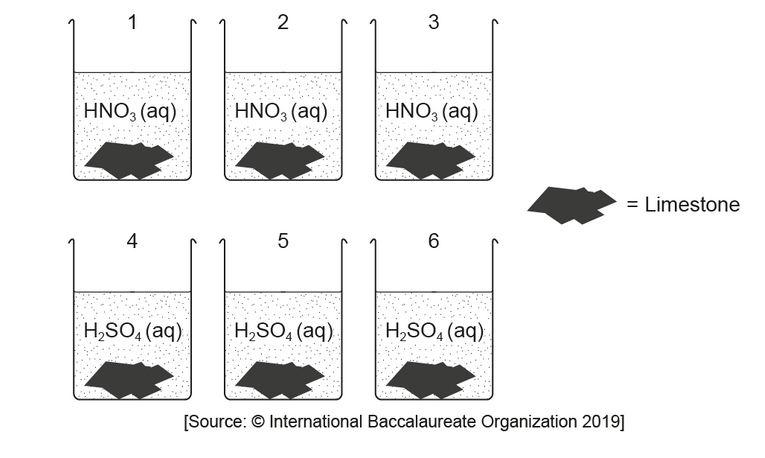
The limestone was removed from the acid, washed, dried with a paper towel and weighed every day at the same time and then replaced in the beakers. The student plotted the mass of one of the pieces of limestone placed in nitric acid against time.
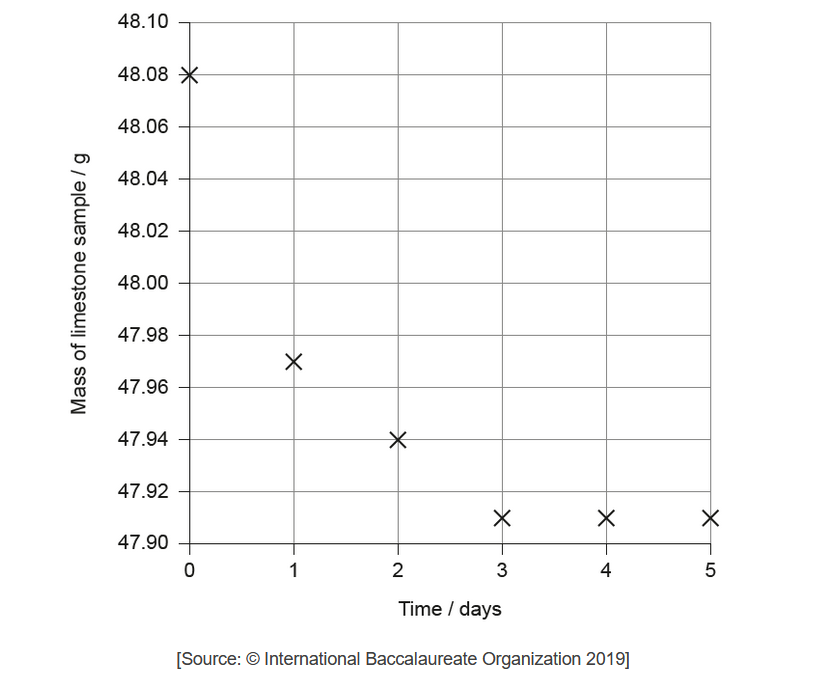
The student hypothesized that sulfuric acid would cause a larger mass loss than nitric acid.
a. Draw a best-fit line on the graph.
Show your working on the graph and include the units of the initial rate.
b(ii Explain why the rate of reaction of limestone with nitric acid decreases and reaches zero over the period of five days.
b(iifuggest a source of error in the procedure, assuming no human errors occurred and the balance was accurate.
c(i)Justify this hypothesis.
c(ii)The student obtained the following total mass losses.
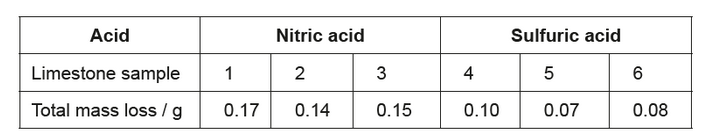
She concluded that nitric acid caused more mass loss than sulfuric acid, which did not support her hypothesis.
Suggest an explanation for the data, assuming that no errors were made by the student.
▶️Answer/Explanation
Markscheme
a. best-fit smooth curve
NOTE: Do not accept a series of connected lines that pass through all points OR any straight line representation.
b(i)tangent drawn at time zero
$\mathrm{g} \mathrm{day}^{-1}$
0.16
M3 can only be awarded if the value corresponds to the correct unit given in $M 2$.
Accept values for the initial rate for M3 in the range: $0.13-0.20 \mathrm{~g} \mathrm{day}^{-1}$ OR $1.5 \times 10^{-6} \mathrm{~g} \mathrm{~s}^{-1}-2.3 \times 10^{-6} \mathrm{~g} \mathrm{~s}^{-1} \mathrm{OR}^{-5} \times 10^{-8}-1.2 \times 10^{-7} \mathrm{~mol} \mathrm{dm}^{-3}$
Ignore any negative rate value.
Award [ 2 max] for answers such as $0.12 / 0.11 \mathrm{~g}$ day $^{-1}$, incorrectly obtained by using the first two points on the graph (the average rate between $t=0$ and 1 day).
Award [1 max] for correctly calculating any other average rate.
b(iipcid used up
OR
acid is the limiting reactant
concentration of acid decreases
OR
less frequent collisions
NOTE: Award [1 max] for “surface area decreases” if the idea that $\mathrm{CaCO}_3$ is used up/acts as the limiting reactant” is conveyed for M1.
Do not accept “reaction reaches equilibrium” for $M 2$.
b(iisįurface area not uniform
NOTE: Accept “acids impure.
OR
limestone pieces do not have same composition/source
NOTE: Accept “«limestone» contains impurities”.
OR
limestone absorbed water «which increased mass»
OR
acid removed from solution when limestone removed
NOTE: Accept “loss of limestone when dried” OR “loss of limestone due to crumbling when removed from beaker”.
OR
«some» calcium sulfate deposited on limestone lost
OR
pieces of paper towel may have stuck to limestone
OR
beakers not covered/evaporation
OR
temperature was not controlled
c(i) sulfuric acid is diprotic/contains two $\mathrm{H}^{+}$«while nitric acid contains one $\mathrm{H}^{+}$»/releases more $\mathrm{H}^{+}$«so reacts with more limestone»
OR
higher concentration of protons $/ \mathrm{H}^{+}$
NOTE: Ignore any reference to the relative strengths of sulfuric acid and nitric acid.
Accept “sulfuric acid has two hydrogens “whereas nitric has one»”.
Accept “dibasic” for “diprotic”.
c(ii)calcium sulfate remained/deposited on limestone «in sulfuric acid»
OR
reaction prevented/stopped by slightly soluble/deposited/layer of calcium sulfate
NOTE: Answer must refer to calcium sulfate.
Question
A student wished to determine the concentration of a solution of sodium hydroxide by titrating it against a 0.100moldm−3 aqueous solution of hydrochloric acid.
4.00g of sodium hydroxide pellets were used to make 1.00dm3 aqueous solution.
20.0cm3 samples of the sodium hydroxide solution were titrated using bromothymol blue as the indicator.
Outline, giving your reasons, how you would carefully prepare the 1.00dm3 aqueous solution from the 4.00g sodium hydroxide pellets.
(i) State the colour change of the indicator that the student would see during his titration using section 22 of the data booklet.
(ii) The student added the acid too quickly. Outline, giving your reason, how this could have affected the calculated concentration.
Suggest why, despite preparing the solution and performing the titrations very carefully, widely different results were obtained.
▶️Answer/Explanation
Markscheme
Key Procedural Steps:
use volumetric flask
mix the solution
fill up to line/mark/«bottom of» meniscus/1 dm3 «with deionized/distilled water»
Key Technique Aspects:
use balance that reads to two decimal places/use analytical balance/use balance of high precision
mix pellets in beaker with deionized/distilled water «and stir with glass rod to dissolve»
use a funnel «and glass-rod» to avoid loss of solution
need to rinse «the beaker, funnel and glass rod» and transfer washings to the «volumetric» flask
Safety Precautions:
NaOH corrosive/reacts with water exothermically
keep NaOH in dessicator
let the solution cool
Two marks may be awarded from two different categories or from within one category.
Do not accept “use of a funnel to transfer the solid”.
Do not accept “keep volumetric flask in cold water/ice”.
(i) blue to green/yellow
(ii) equivalence point has been exceeded
OR
greater volume of/too much acid has been added
«calculated» concentration increased
Accept “end-point” for “equivalence point”.
colour difficult to detect
OR
using different HCl standards
OR
no significant figures used in subsequent calculation
OR
incorrect method of calculation
Accept any valid hypothesis.
Do not accept any mistakes associated with techniques (based on stem of question) eg. parallax error, not rinsing glassware, etc.
Do not accept “HCl was not standardized”.
Accept “reaction of NaOH with CO2 «from air»”.
Accept “NaOH hygroscopic/absorbs moisture/H2O «from the air/atmosphere»”.
Accept “impurities in NaOH”.
Accept “temperature changes during experiment”.
Ignore a general reference to random errors.
Question
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
Calculate the percentage uncertainty of the volume of the aqueous sodium hydroxide.
Suggest how the precision of this measurement could be improved.
▶️Answer/Explanation
Markscheme
«\(\frac{{0.5}}{{25.0}} \times 100\)» = 2 «%»
[1 mark]
pipette/pipet «rather than a measuring cylinder»
Accept “using a burette/buret”.
Accept “using a volumetric/measuring flask”.
Do not accept “use of a more precise measuring cylinder”.
[1 mark]
Question
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
The graph of temperature against titre can be used to calculate the concentration of alkali without knowing the concentration of the hydrochloric acid, using the enthalpy of neutralization.
Explain how the concentration may be calculated in this way.
Heat losses would make this method less accurate than the pH probe method. Outline why the thermometric method would always give a lower, not a higher, concentration.
Suggest how heat loss could be reduced.
State one other assumption that is usually made in the calculation of the heat produced.
Suggest why scientists often make assumptions that do not correspond to reality.
Outline why the thermochemical method would not be appropriate for 0.001 mol\(\,\)dm−3 hydrochloric acid and aqueous sodium hydroxide of a similar concentration.
▶️Answer/Explanation
Markscheme
heat change/evolved can be calculated from the «maximum» temperature increase and the mass of solution
OR
q = mcΔT
heat «evolved» gives the number of moles «of both acid and alkali present when neutralisation occurs»
OR
\(n = \frac{q}{{\Delta {H_{neut}}}}\)
volume «of acid and the volume of alkali required to just neutralise each other» can be used to calculate the concentration«s of both»
OR
\(\left[ {{\text{NaOH}}} \right] = \frac{n}{V}\)
[2 marks]
smaller temperature increase/ΔT
OR
heat released would «appear to» be less
amount of substance/n calculated is smaller
[2 marks]
using «expanded» polystyrene cup
OR
insulating beaker
OR
putting a lid on beaker
Do not accept calorimeter by itself.
Accept any other reasonable suggestion.
[1 mark]
«specific» heat capacity of the beaker/container/thermometer is ignored
OR
density of the solutions is assumed as 1.00 g\(\,\)cm–3/same as water
OR
specific heat capacity of the solutions is assumed as 4.18 J g–1\(\,\)K–1/same as water
Accept “reaction goes to completion”.
Accept “reaction is conducted under standard conditions”.
Accept “no evaporation occurs”.
Accept any other relevant valid assumption.
Do not accept “heat is not released from other reactions”.
[1 mark]
allows simple theories to be applied to real life situations
OR
enables us to start to understand complex situations
OR
gives answers that are accurate to the required order of magnitude
OR
simplifies the calculations involved
Do not accept “to simplify the situation” without further detail.
Accept “errors do not have a major impact on the results”.
[1 mark]
temperature rise would be too small «to be accurately measured»
Accept “heat released would be too small «to be accurately measured»”.
[1 mark]
Question
There is a link between world energy consumption and carbon dioxide production.
Climate induced changes in the ocean can be studied using measurements such as the Atmospheric Potential Oxygen (APO). Trends in APO concentration from two stations, one in each hemisphere, are shown below.
Trends in atmospheric potential oxygen (APO) based on monthly averages between 1990 and 2010.
[Source: www.ioos.noaa.gov]
The following graph represents world energy consumption by type for the years 1988–2013.
Estimate the percentage of energy consumption which did not directly produce CO2 in 2013.
O2 is consumed in producing CO2 for electricity generation. The graph shows the relationship between the world’s electricity generation and CO2 production between 1994 and 2013.
Calculate the mass, in million tonnes, of oxygen gas ultimately found in CO2 which is consumed in generating 18\(\,\)000 terawatts of electricity using the equation given for the best fit line. Give your answer to 2 significant figures.
Assume coal is the only energy source.
The equilibrium expression for O2 exchange between the atmosphere and ocean is O2(g) \( \rightleftharpoons \) O2(aq). Identify one factor which shifts the equilibrium to the right.
Factors such as photosynthesis and respiration are excluded so that APO is influenced by oceanic changes only. Suggest why the seasonal cycles from Alert station and Cape Grim observatory are different.
The change in APO O2/N2 ratio, per meg, is measured relative to an O2/N2 reference.
\[\Delta {\text{(}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{N}}_{\text{2}}}{\text{)}} = \left( {\frac{{{{({{\text{O}}_2}/{{\text{N}}_2})}_{{\text{sample}}}}}}{{{{({{\text{O}}_2}/{{\text{N}}_2})}_{{\text{reference}}}}}} – 1} \right) \times {10^6}\]
Calculate the APO Δ(O2/N2) value for an oxygen concentration of 209\(\,\)400 ppm assuming that any change in N2 concentration is negligible. Reference values for O2 and N2 are 209 460 and 790 190 ppm respectively.
Suggest a reason for the general negative gradient of the APO curve given in (c).
▶️Answer/Explanation
Markscheme
«\(\frac{{\sum {({\text{renewables}} + {\text{hydroelectricity}} + {\text{nuclear)}}} }}{{{\text{total}}}}\)»
«\(\left( {\frac{{8800 – 7200}}{{12600}}} \right) \times 100 = \)» 13 «%»
Accept range of “11–16%”.
[1 mark]
«18000 = 0.54x – 2000»
x = 37037 «million tonnes of CO2»
«\(\frac{{32.00}}{{44.01}}\) x 37037 = 26930»
27000/2.7 x 104 «million tonnes of O2»
Accept “37000 «million tonnes of CO2»” for M1.
Award [2] for correct final answer with two significant figures.
Award [1] for non rounded answers in range 26903–26936 «million tonnes of O2».
[2 marks]
increase in «atmospheric» pressure
OR
increase in [O2(g)]/concentration of O2(g)
OR
decrease in [O2(aq)]/concentration of O2(aq)
OR
decrease in temperature
Accept “increase in volume of oceans «due to polar ice cap melting»” OR “consumption of O2 in oceans/O2(aq)
«by living organisms»”.
State symbols required for oxygen concentration.
[1 mark]
summer in one station while winter in other
OR
stations are at different latitudes
oxygen dissolves better in colder water
Accept “opposite seasons «in each hemisphere»”.
Do not accept “different locations with different temperatures” OR “stations are in different hemispheres”.
[2 marks]
«\({\text{(}}\frac{{209400}}{{209460}} – 1{\text{)}} \times {10^6}\) =» − 286.5 «per meg»
The nitrogen cancels so is not needed in the calculation.
Negative sign required for mark.
[1 mark]
decrease in [O2]/concentration of O2
OR
increasing combustion of fossil fuels «consumes more O2 so [O2]/concentration of O2 decreases»
OR
warmer oceans/seas/water «as oxygen dissolves better in colder water»
OR
deforestation
Accept “decrease in level of O2”.
Accept “increasing CO2 production «consumes more O2 so [O2]/concentration of O2 decreases»”.
Do not accept “decrease in amount of O2” OR “increase in greenhouse gases”.
[1 mark]
Question
Antacids react with hydrochloric acid in the stomach to relieve indigestion. A student investigated different brands of antacid to see which caused the largest increase in pH in a given time. She added the antacids to hydrochloric acid, and recorded the change in pH over five minutes.
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
Suggest two variables, besides the time of reaction, which the student should have controlled in the experiment to ensure a fair comparison of the antacids.
Calculate the uncertainty in the change in pH.
The student concluded that antacid B was the most effective, followed by A then C and finally D. Discuss two arguments that reduce the validity of the conclusion.
▶️Answer/Explanation
Markscheme
Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O (l)
Accept full or net ionic equation.
Any two from:
volume «of HCl»
concentration «of HCl»/[HCl]
temperature «of HCl»
mass of antacid/tablets
size of antacid particles/tablets
OR
surface area of antacid «particles»/tablets
Accept “number of tablets/different doses”.
Do not accept “same pH meter” OR “initial pH” OR “concentration of antacid/[antacid]”.
A variable must be given so do not accept answers such as “stirring”, “whether tablets are whole or crushed” etc.
[Max 2 Marks]
(±) 0.04
OR
(±) 0.03
Any two of:
uncertainty «(±)0.04/(±)0.03» means A and C cannot be distinguished
each measurement was conducted once
stomach pH should not be raised a lot «so antacid B is not necessarily effective»
mass/number of tablets/dose «of antacid» used was not controlled
actual environment in stomach is different
Accept “amount of tablets” for “dose”.
Do not accept “nature/composition of tablets differs”.
Accept an answer such as “time frame is too short since some antacids could be long-acting drugs if they contain a gelatinisation/delaying agent” but not just “time frame is too short since some antacids could be long-acting drugs”.
[Max 2 Marks]
Question
Disposable plastic lighters contain butane gas. In order to determine the molar mass of butane, the gas can be collected over water as illustrated below:
List the data the student would need to collect in this experiment.
Explain why this experiment might give a low result for the molar mass of butane.
Suggest one improvement to the investigation.
▶️Answer/Explanation
Markscheme
mass/m of lighter before AND after the experiment
volume of gas/Vgas «collected in the cylinder»
«ambient» pressure/P «of the room»
temperature/T
Accept “change in mass of lighter”.
Accept “weight” for “mass”.
Do not accept just “mass of lighter/gas”.
Accept “volume of water displaced”.
Do not accept “amount” for “volume” or “mass”.
[4 marks]
Any two of:
pressure of gas not equalized with atmospheric/room pressure
too large a recorded volume «of gas produces a lower value for molar mass of butane»
OR
cylinder tilted
difficult to dry lighter «after experiment»
OR
higher mass of lighter due to moisture
OR
smaller change in mass but same volume «produces lower value for molar mass of butane»
using degrees Celcius/°C instead of Kelvin/K for temperature
Accept “vapour pressure of water not accounted for” OR “incorrect vapour pressure of water used” OR “air bubbles trapped in cylinder”. Do not accept “gas/bubbles escaping «the cylinder»” or other results leading to a larger molar mass.
Accept “lighter might contain mixture of propane and butane”.
Do not accept only “human errors” OR “faulty equipment” (without a clear explanation given for each) or “mistakes in calculations”.
[2 marks]
record vapour pressure of water «at that temperature»
OR
equalize pressure of gas in cylinder with atmospheric/room pressure
OR
tap cylinder before experiment «to dislodge trapped air»
OR
collect gas using a «gas» syringe/eudiometer/narrower/more precise graduated tube
OR
collect gas through tubing «so lighter does not get wet»
OR
dry lighter «before and after experiment»
OR
hold «measuring» cylinder vertical
OR
commence experiment with cylinder filled with water
Accept “adjust cylinder «up or down» to ensure water level inside cylinder matches level outside”.
Accept “repeat experiment/readings «to eliminate random errors»”.
Accept “use pure butane gas”.
[1 mark]
Question
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
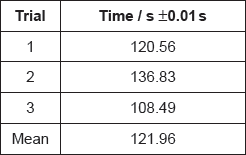
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
▶️Answer/Explanation
Markscheme
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Accept “CO2(aq)”.
[1 mark]
measure the volume of gas at different times «plot a graph and extrapolate»
OR
measure the mass of the reaction mixture at different times «plot a graph and extrapolate»
Accept other techniques that yield data which can be plotted and extrapolated.
[1 mark]
method 2 AND marble is in excess «so a little extra has little effect»
OR
large chips AND marble is in excess «so a little extra has little effect»
OR
method 2 AND HCl is limiting reagent «so a little extra marble has little effect»
OR
large chips AND HCl is limiting reagent «so a little extra marble has little effect»
Accept, as a reason, that “as the mass is greater the percentage variation will be lower”.
[1 mark]
surface area
OR
purity «of the marble»
Accept “shape of the chip”.
[1 mark]
variation of individual values is much greater «than this uncertainty»
OR
«uncertainty» does not take into account «student» reaction time
[1 mark]
«\(\frac{{121.96{\text{ s}}}}{2}\) = 60.98 s» = 61 «s»
[1 mark]
systematic AND always makes the time shorter «than the actual value»
OR
systematic AND it is an error in the method used «not an individual measurement»
OR
systematic AND more repetitions would not reduce the error
Accept, as reason, “it always affects the value in the same direction” OR “the error is consistent”.
[1 mark]

