Question
Lithium has many uses.
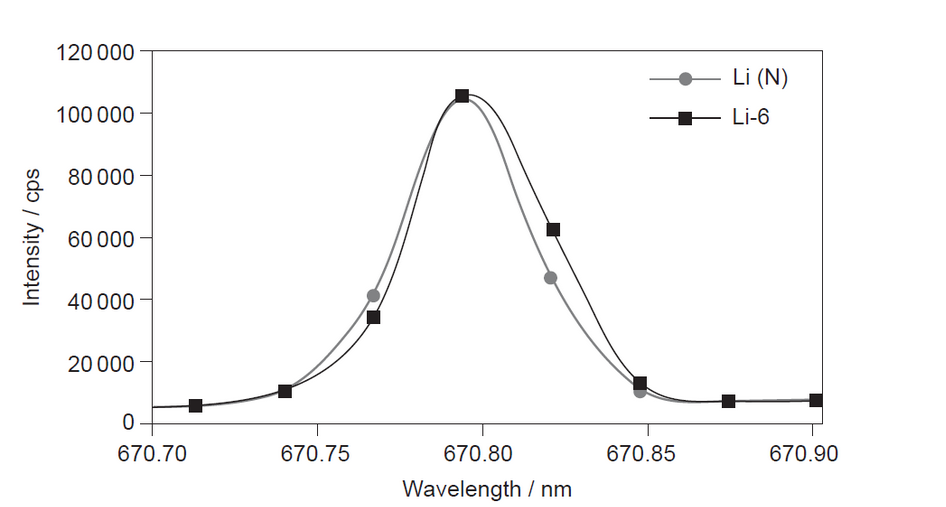
The emission spectra obtained by ICP-OES for a mixture containing the isotope ${ }^6 \mathrm{Li}(\mathrm{Li}-6)$ and naturally occurring lithium (Li (N)) is shown.
a. Identify the type of bonding in lithium hydride, using sections 8 and 29 of the data booklet.
b(i)Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
b(iiß) Sugest why ICP-OES does not give good quantitative results for distinguishing ${ }^6$ Li from naturally occurring lithium.
b(iiisuggest a better method.
c. Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit $0.694 \mathrm{~g} \mathrm{Li}$ using a current of $2.00 \mathrm{~A}$.
$$
Q(\text { charge })=I(\text { current }) \times t(\text { time })
$$
d(i)Lithium has shown some superconductive properties when doped into graphene or when under high pressure. Under high pressure, however, the Meissner effect is absent.
Describe the Meissner effect.
$\mathrm{d}($ (ii)At very low temperatures, lithium atoms enhance the phonon binding of electrons in graphene suggesting the formation of Cooper pairs. Explain how Cooper pairs are formed.
e. Lithium forms a crystalline lattice with the unit cell structure shown below.
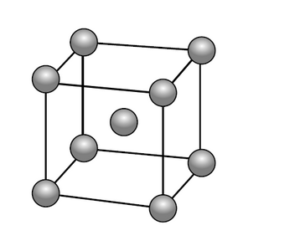
X-ray diffraction shows that the length of the edge of the unit cell is $3.51 \times 10^{-8} \mathrm{~cm}$.
Determine the density of lithium, in $\mathrm{g} \mathrm{cm}^{-3}$, using sections 2 and 6 of the data booklet.
▶️Answer/Explanation
Markscheme
a. ionic [ ]
b(ii)mission spectra of both « ${ }^6 \mathrm{Li}$ and natural Li» give same colour/produce same «range of» wavelengths
OR
they have same electron transitions/same nuclear charge $\quad[\checkmark]$
Note: Accept “the spectra are almost identical”.
b(iii)CP-MS [ $\boldsymbol{V}]$
Note: Accept “MS/mass spectrometry”.
$$
\begin{aligned}
& \text { «t }=\frac{0.100 \mathrm{~mol} \times 96500 \mathrm{C} \mathrm{mol}^{-1}}{2.00 \mathrm{C} \mathrm{s}^{-1}} » \\
& 4830 \text { «s»[} \boldsymbol{J}]
\end{aligned}
$$
$\mathrm{d}(\mathrm{i})$ creation of mirror image/opposing magnetic field of external field «below critical temperature/T of superconductor»
OR
expulsion of magnetic field from superconductor «below critical temperature/T» [ $[\boldsymbol{J}]$
d(ii)Any three of:
positive ions/cations in lattice are attracted to passing electron [ $\checkmark$ ]
lattice is distorted «by this passing electron» $[\boldsymbol{\sim}]$
creates «local» regions of increased positive charge $\quad[\boldsymbol{V}]$
second electron is attracted to deformation AND a coupling occurs
$[\boldsymbol{c ]}$
e. mass of Li in unit cell $=« 2 \times \frac{6.94 \mathrm{~g} \mathrm{~mol}^{-1}}{6.02 \times 10^{23} \mathrm{~mol}^{-1}} » 2.31 \times 10^{-23} \mathrm{~g} \quad$ [ $]$
volume of unit cell $=«\left(3.51 \times 10^{-8} \mathrm{~cm}\right)^3=» 4.32 \times 10^{-23} \mathrm{~cm}^3 \quad$ [ $]$
«density $=\frac{2.31 \times 10^{-23} \mathrm{~g}}{4.32 \times 10^{-23} \mathrm{~cm}^3}=» 0.535 \ll \mathrm{g} \mathrm{cm}^{-3} » \quad[\mathscr{U}]$
Note: Award [3] for correct final answer.
Question
The complex ion \({{\text{[Ni(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) is green and \({{\text{[Ni(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) is blue. Explain why the \({{\text{[Ni(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) complex ion is coloured and outline why changing the identity of the ligand changes the colour of the ion.
▶️Answer/Explanation
Markscheme
Explanation of colour:
d orbitals splits (into two levels);
due to repulsion between d electrons and non-bonding electrons on ligand / due to interaction with electric field of ligands;
difference in energy between levels corresponds to visible light;
(visible light absorbed as) electrons move from lower to higher energy d orbitals;
colour observed complementary to absorbed;
Why changing ligand changes colour:
more electron-dense ligand greater splitting of d orbitals;
3 NH ligand has greater (crystal field/ligand) splitting energy / NH3 ligand at higher energy in spectrochemical series / OWTTE;
Accept “changing ligand changes d-orbital splitting”.
Examiners report
Surprisingly this was very poorly answered and few students scored all four marks here. There was often no clear understanding of the mechanism of colour absorption (and hence the observed colour being complementary – many candidates thought that the colour was caused by the subsequent emission of energy by electrons falling back). Some of the better candidates did refer to the fact that the ammonia ligand has a greater crystal field/ligand field splitting, which clearly showed comprehensive understanding of this subject.
Question
When excess ammonia solution is added to a solution of copper(II) sulfate the oxidation number of the copper ion does not change but there is a noticeable colour change. Outline the reasons for this change in colour.
▶️Answer/Explanation
Markscheme
the \({{\text{H}}_{\text{2}}}{\text{O}}\) ligand is exchanged for \({\text{N}}{{\text{H}}_{\text{3}}}\);
Accept suitable equation with co-ordination numbers of 4 and 6.
colour is due to electron transitions between the split d orbitals;
\({\text{N}}{{\text{H}}_{\text{3}}}\) causes a greater/different splitting than \({{\text{H}}_{\text{2}}}{\text{O}}\);
Examiners report
Quite a few candidates referred to electron transition between split d-orbitals, but they generally did not realize that the exchange of the ligand was the reason for the change in colour, and so lost 2 marks.
