Question
a.i.Define the term isotopes.[1]
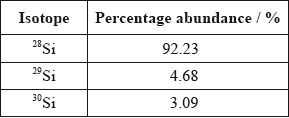
Calculate the relative atomic mass of silicon using this data.[2]
▶️Answer/Explanation
Markscheme
a.i. atoms of the same element with the same atomic number/Z/same number of protons, but different mass numbers/A/different number of neutrons;
\((0.9223 \times 28) + (0.0468 \times 29) + (0.0309 \times 30)\);
28.1/28.11;
Working must be shown to get [2], do not accept 28.09 on its own (given in the data booklet).
Silicon dioxide
single covalent (bonds);
network/giant covalent/ macromolecular / repeating tetrahedral units;
a.iv.
Carbon dioxide
double covalent (bonds);
(simple / discrete) molecular;
Marks may be obtained from suitable structural representations of SiO2 and CO2.
 ;
;
Allow crosses or dots for lone-pair.
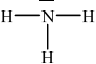
trigonal/triangular pyramidal;
b.ii (\( \sim \))107° / less than 109.5°;
Do not allow ECF.
LP-BP repulsion \( > \) BP-BP repulsion / one lone pair and three bond pairs / lone pairs/non-bonding pairs repel more than bonding-pairs;
Do not accept repulsion between atoms.
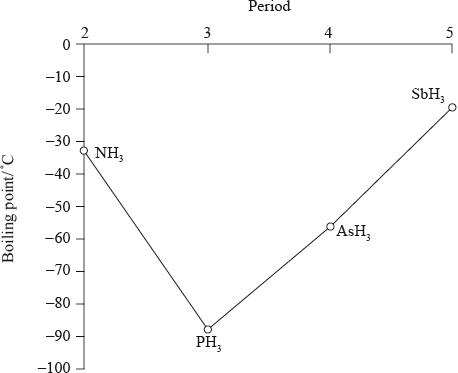
boiling points increase going down the group (from \({\text{P}}{{\text{H}}_{\text{3}}}\) to \({\text{As}}{{\text{H}}_{\text{3}}}\) to \({\text{Sb}}{{\text{H}}_{\text{3}}}\));
\({M_{\text{r}}}\)/number of electrons/molecular size increases down the group;
Accept electron cloud increases down the group for the second marking point.
greater dispersion/London/van der Waal’s forces;
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia has a higher boiling point than expected due to the hydrogen bonding between the molecules;
Do not accept hydrogen bonding alone.
CO:
![]()
Award [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
c.ii. \(N{O_2}\):
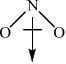
Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
c.iii. \(C{O_2}\):
![]()
Award [1] for correct representation of the linear shape and [1] for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For all three molecules, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
Detailed Solution:
a.
i. Isotopes are variants of an element that have the same number of protons in the nucleus but differ in the number of neutrons. In other words, isotopes are different forms of an element that have the same atomic number (number of protons) but different atomic masses (sum of protons and neutrons).
ii. Relative atomic mass = (Sum of the product of isotope mass and fraction abundance)
Relative atomic mass = 28*0.0223+29*0.0468+30*0.0309 = 28.1.
iii.
Silicon dioxide (SiO2) is a chemical compound commonly known as silica or quartz. It is a covalent compound that consists of one silicon atom bonded to two oxygen atoms. The structure of silicon dioxide can be described as a three-dimensional network of interconnected silicon and oxygen atoms.
In the crystal lattice of silicon dioxide, each silicon atom is surrounded by four oxygen atoms in a tetrahedral arrangement. Each oxygen atom is shared between two silicon atoms, forming strong covalent bonds. This results in a highly stable and rigid structure.
![]()
iv.
The structure of carbon dioxide (CO2) can be described as a linear molecule with a carbon atom in the center bonded to two oxygen atoms. Each oxygen atom is connected to the carbon atom by a double bond.
In the Lewis structure representation, the carbon atom is in the middle and has two electron pairs and two oxygen atoms around it. Each oxygen atom shares two electrons with the carbon atom through a double bond, resulting in a total of four shared electrons. The remaining two electron pairs on each oxygen atom are unshared (also known as lone pairs).
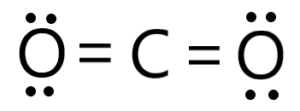
b.
i.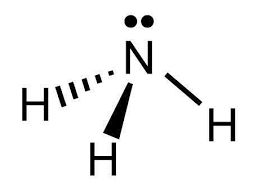
The shape of ammonia (NH3) is trigonal pyramidal.
In ammonia, the central nitrogen atom is bonded to three hydrogen atoms. The nitrogen atom has one lone pair of electrons, in addition to the three bonding pairs of electrons. The three hydrogen atoms are positioned around the nitrogen atom, forming a triangular base.
The lone pair of electrons on nitrogen affects the molecular geometry, pushing the bonding electron pairs slightly closer to each other. This results in a distorted tetrahedral arrangement, where the three hydrogen atoms and the lone pair of electrons are not symmetrically arranged around the nitrogen atom.
ii. The bond angles between the hydrogen-nitrogen-hydrogen atoms are approximately 107 degrees, slightly less than the ideal tetrahedral angle of 109.5 degrees, due to the repulsion between the lone pair and bonding pairs of electrons.
iii.
PH3 < NH3
The lower boiling point of ammonia compared to phosphine is primarily due to the differences in molecular polarity and hydrogen bonding.
Ammonia (NH3) has a higher boiling point than expected based on its molecular weight alone because it can form hydrogen bonds. In ammonia, the electronegative nitrogen atom can attract the hydrogen atoms of neighboring molecules, resulting in intermolecular hydrogen bonding. These hydrogen bonds are relatively strong and require more energy to break, resulting in a higher boiling point for ammonia.
Phosphine (PH3), on the other hand, is less polar and exhibits weaker intermolecular forces compared to ammonia. While phosphine molecules can form hydrogen bonds, the electronegativity difference between phosphorus and hydrogen is smaller than that between nitrogen and hydrogen. As a result, the strength of hydrogen bonding in phosphine is weaker than in ammonia, leading to a lower boiling point for phosphine.
PH3 < AsH3 < SbH3
PH3 (Phosphine): Phosphine molecules have a dipole moment due to the electronegativity difference between phosphorus and hydrogen. However, the dipole moment in phosphine is relatively weak. Additionally, phosphine experiences relatively weak London dispersion forces between molecules. As a result, the intermolecular forces in phosphine are weaker overall, leading to a lower boiling point compared to the other compounds.
AsH3 (Arsine): Arsine molecules have a larger dipole moment compared to phosphine due to the electronegativity difference between arsenic and hydrogen. This increased dipole moment enhances the strength of dipole-dipole interactions between arsine molecules. As a result, arsine has stronger intermolecular forces compared to phosphine, leading to a higher boiling point.
SbH3 (Stibine): Stibine molecules have an even larger dipole moment than arsine due to the electronegativity difference between antimony and hydrogen. The increased dipole moment in stibine further enhances the strength of dipole-dipole interactions compared to arsine. Additionally, stibine experiences stronger London dispersion forces due to the larger, more polarizable antimony atom. These stronger intermolecular forces result in a higher boiling point for stibine compared to both arsine and phosphine.
c.
i. ![]()
CO (carbon monoxide) is a polar molecule due to the difference in electronegativity between carbon and oxygen atoms and the molecular geometry.
In a CO molecule, oxygen is more electronegative than carbon, meaning it has a higher affinity for electrons. As a result, the shared electrons in the carbon-oxygen bond are pulled more towards the oxygen atom, creating a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the carbon.
Additionally, the CO molecule has a linear geometry, with carbon in the center and oxygen on one side. Since the oxygen atom has a higher electronegativity, the polar bond moments (represented by arrows pointing towards the more electronegative oxygen atom) do not cancel each other out. Instead, they result in a net dipole moment along the carbon-oxygen bond.
ii. 
NO2 (nitrogen dioxide) is a polar molecule due to the presence of a polar bond and an asymmetrical molecular geometry. In a NO2 molecule, nitrogen (N) and oxygen (O) are bonded together by a covalent bond. Oxygen is more electronegative than nitrogen, which means it has a higher affinity for electrons. As a result, the shared electrons in the nitrogen-oxygen bond are pulled more towards the oxygen atom, creating a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the nitrogen.
Furthermore, the NO2 molecule has a bent or V-shaped geometry. This shape arises due to the repulsion between the lone pair of electrons on the nitrogen atom and the bonding electron pairs. The lone pair pushes the oxygen atoms closer together, resulting in an asymmetrical distribution of charge.Due to the presence of a polar bond (N-O) and the asymmetrical molecular geometry, the individual bond dipoles do not cancel each other out. Instead, they combine to give the molecule a net dipole moment.
iii.![]()
CO2 (carbon dioxide) is a nonpolar molecule due to its symmetrical linear molecular geometry and the cancellation of dipole moments.
In a CO2 molecule, carbon is bonded to two oxygen atoms through double bonds. Carbon and oxygen have different electronegativities, with oxygen being more electronegative. However, the molecule’s linear geometry ensures that the bond dipoles between carbon and each oxygen are equal in magnitude but opposite in direction.
The two oxygen atoms are positioned on opposite sides of the carbon atom, resulting in a linear arrangement. Since the dipole moments of the two carbon-oxygen bonds are equal and pointing in opposite directions, they cancel each other out. This cancellation of dipole moments leads to a net dipole moment of zero, making CO2 a nonpolar molecule.
