Question
1. Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
Define the terms
(i)Atomic number:
(ii)Mass number:
(iii)Isotopes of an element:
(iv)Distinguish between the terms group and period.
Deduce the electron arrangements of:
(v) \({\text{L}}{{\text{i}}^ + }\):
(vi) B:
(vii)Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for (viii) Li-6 and (ix) Li-7 isotope.
 [10]
[10]
Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the (ii) Ultraviolet and (iii) Visible regions of the spectrum. Label your diagram to show three transitions for each series.[6]
(iii) Iron is described as a transition metal. Identify the two most common ions of iron.
c.Deduce the chemical formulas of
(i)Lithium oxide:
(ii) Iron(II) oxide: [4]
▶️Answer/Explanation
Markscheme
(i) Atomic number:
number of protons (in nucleus/atom);
ii. Mass number:
(sum of) number of protons and neutrons (in nucleus/atom);
iii. Isotopes of an element:
atoms of same element / atoms with same number of protons/atomic number/Z but different number of neutrons/mass number/A;
Penalize once only use of the term element in the three definitions, for example, number of protons in an element or number of protons and neutrons in an element or element with the same atomic number but different mass number.
(iv) Group: (elements in vertical) columns in periodic table and Period: (elements in horizontal) rows in periodic table;
Allow elements in same group have similar chemical properties and within a period, atoms have same number of shells/energy levels (but number of electrons in valence/outer shell increases).
Allow groups distributed vertically and periods distributed horizontally / OWTTE.
Allow group number gives number of valence/outer shell electrons (for maingroup elements) and period gives same number of shells/energy levels.
(v) Li+: 2/1s2;
(vi) B: 2,3/1s22s22p1;
(vii) correct mathematical expression set-up \({\text{(e.g. }}\left( {\frac{x}{{100}}} \right)(10) + \left[ {\frac{{(100 – x)}}{{(100)}}} \right](11) = 10.81)\);
19%;
Award [2] for correct final answer.
(viii) 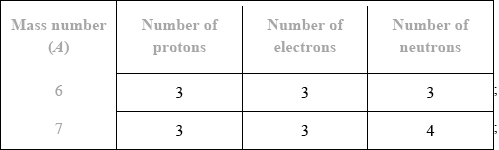
Award [1 max] for correct number of neutrons for both isotopes if numbers of protons or electrons is not given.
Award [1 max] for correct number of protons and electrons for both isotopes if number of neutrons is not given or if numbers of neutrons are incorrect.
(i) Continuous spectrum: radiation spread over all wavelengths/frequencies/energies/colours / OWTTE;
Line spectrum: radiation (absorbed/emitted) at certain/specific wavelengths/frequencies/energies/colours / OWTTE;
Allow series of (separate/discrete) lines which converge/get closer together at high energy / OWTTE.
(ii) 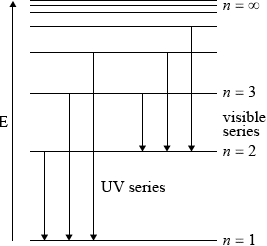
showing y-axis labelled as energy/E or labelling at least two energy levels
(\(n = 1\), \(n = 2\) etc. but not for \(n = 0\));
showing energy levels converging;
showing jumps to \(n = 1\) for ultraviolet series;
(iii) showing jumps to \(n = 2\) for visible series;
UV and visible must be labelled.
(i) metals have delocalized electrons / sea of electrons which are mobile/can move / OWTTE;
(ii)layers/positive ions/cations/atoms slide past/over each other / OWTTE;
Do not accept nuclei for M2.
(iii) Fe2+and Fe3+;
c.
(i) Lithium oxide: Li2O and
(ii) Iron(II) oxide: FeO;
Detailed Solution:
1.
(i) Atomic number: The atomic number of an element represents the number of protons found in the nucleus of an atom of that element. It is denoted by the symbol “Z” and is a unique characteristic of each element. The atomic number determines the identity of an element and its position on the periodic table.
(ii) Mass number: The mass number of an atom is the sum of the number of protons and neutrons present in the nucleus. It is denoted by the symbol “A”. The mass number provides an approximate measure of the total mass of an atom and is used to identify different isotopes of an element.
(iii) Isotopes: Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei. While isotopes have the same atomic number (number of protons) because they belong to the same element, they have different mass numbers due to the varying number of neutrons. Isotopes share similar chemical properties but may exhibit different physical properties, such as different atomic masses and radioactive behavior.
(iv) Group: A group, also known as a family, is a vertical column in the periodic table. Elements within the same group share similar chemical properties because they have the same number of valence electrons, which are the electrons in the outermost energy level of an atom. The group number indicates the number of valence electrons for elements in that group. For example, Group 1 elements, such as hydrogen (H) and sodium (Na), all have one valence electron.
Period: A period is a horizontal row in the periodic table. Elements in the same period do not necessarily share similar properties. Instead, the period number indicates the number of electron shells or energy levels occupied by the electrons in the atom. Each period represents a new energy level being filled with electrons.
(v)
The electronic arrangement of Li+ (lithium ion) can be determined by removing one electron from the neutral lithium atom (Li).
The electron configuration of a neutral lithium atom is 1s2 2s1.
By removing one electron, the electronic arrangement of Li+ becomes 1s2.
Since the lithium ion has lost one electron, it now has a filled 1s orbital and no valence electrons. The removal of the valence electron results in the formation of the lithium ion with a positive charge (Li+).
(vi) Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s22s22p1.
(vii) Relative atomic mass = Sum of product of isotope mass and fraction abundance
Relative atomic mass of Boron = 10.81
Let fraction of B-10 is x and B-11 is (1-x).
10.81 = 10x+11(1-x)
x=0.19
B-10 has 19% bundance.
(viii)
Lithium-6:
Number of protons: The atomic number of lithium is 3, which indicates that it has 3 protons.
Number of electrons: In a neutral atom, the number of electrons is equal to the number of protons. Therefore, lithium-6 also has 3 electrons.
Number of neutrons: To determine the number of neutrons, we subtract the atomic number (number of protons) from the mass number. For lithium-6, the mass number is 6, and since it has 3 protons, the number of neutrons would be 6 – 3 = 3.
So, lithium-6 has 3 protons, 3 electrons, and 3 neutrons.
(ix)
Lithium-7:
Number of protons: The atomic number of lithium is still 3, so lithium-7 also has 3 protons.
Number of electrons: Similar to lithium-6, lithium-7, as a neutral atom, has 3 electrons.
Number of neutrons: By subtracting the atomic number (3) from the mass number (7), we find that lithium-7 has 7 – 3 = 4 neutrons.
Thus, lithium-7 has 3 protons, 3 electrons, and 4 neutrons.
a.(i) A continuous spectrum and a line spectrum are two different types of spectra observed in the study of light emitted or absorbed by atoms or molecules. Here’s how they differ:
Continuous Spectrum: A continuous spectrum, also known as a continuous emission or absorption spectrum, is characterized by a continuous range of wavelengths or colors. It covers a broad range of wavelengths without any gaps or missing wavelengths. In a continuous spectrum, light is emitted or absorbed by a substance over a wide range of energies. An example of a continuous spectrum is the light emitted by an incandescent lamp or sunlight.
Line Spectrum: A line spectrum, also called a discrete spectrum or atomic spectrum, consists of only specific and distinct wavelengths or colors. It consists of individual lines or bands of light at specific wavelengths. Each line in a line spectrum corresponds to a particular energy transition within an atom or molecule. Line spectra are often observed when atoms or molecules emit or absorb light in a quantized manner. The most famous example is the emission spectrum of hydrogen, which consists of a series of discrete lines at specific wavelengths corresponding to different electron transitions within hydrogen atoms.
(ii) 
Lyman Series (Ultraviolet): The Lyman series corresponds to electron transitions to the n = 1 energy level. When an electron drops from higher energy levels to the n = 1 level, it emits photons in the ultraviolet region. The spectral lines in the Lyman series are not visible to the naked eye. The first line in the Lyman series (n = 2 to n = 1 transition) corresponds to the Lyman-alpha line at 121.6 nm.
(iii) Balmer Series (Visible): The Balmer series corresponds to electron transitions to the n = 2 energy level. When an electron drops from higher energy levels to the n = 2 level, it emits photons in the visible region of the electromagnetic spectrum. The Balmer series includes several visible spectral lines. The first line in the Balmer series (n = 3 to n = 2 transition) corresponds to the H-alpha line at 656.3 nm, which appears as red light.
b.
(i)Metals are good conductors of electricity due to the presence of delocalized electrons and their unique crystal structure. Here’s why metals exhibit high electrical conductivity: Delocalized electrons: In metallic bonding, outer electrons of metal atoms are loosely held and can move freely throughout the metal lattice. These electrons are not bound to a specific atom and are called delocalized electrons. They form an electron sea or cloud that can flow in response to an electric field. This mobility of delocalized electrons allows metals to conduct electricity efficiently.
(ii) Metals are malleable, meaning they can be easily hammered or bent into various shapes, due to the following factors:
Metallic bonding: In metals, the atoms are arranged in a closely-packed lattice structure. The positive metal ions are surrounded by a sea of delocalized electrons, which act as a binding force. The metallic bonds between positive ions and the shared electrons are strong, but not directional, allowing layers of atoms to slide past each other without breaking the bonds completely.
Plastic deformation: When an external force, such as hammering or pressure, is applied to a metal, the layers of metal ions can slide and shift relative to each other. This ability of metal atoms to move and retain their metallic bonding makes metals malleable. The delocalized electrons facilitate the movement of metal ions and maintain the integrity of the metal lattice.
(iii)Iron (Fe) is indeed classified as a transition metal. The two most common ions of iron are:
Iron(II) ion: The iron(II) ion is formed when iron loses two electrons. It has a +2 charge and is represented as Fe2+. This ion is also known as ferrous ion.
Iron(III) ion: The iron(III) ion is formed when iron loses three electrons. It has a +3 charge and is represented as Fe3+. This ion is also known as ferric ion.
c.
(i) Lithium oxide:
Lithium (Li) has a charge of +1, and oxygen (O) has a charge of -2. To balance the charges, we need two lithium ions to combine with one oxygen ion. Therefore, the chemical formula for lithium oxide is Li2O.
(ii) Iron(II) oxide:
Iron(II) (Fe2+) has a charge of +2, and oxygen (O) has a charge of -2. To balance the charges, we need one iron(II) ion to combine with one oxygen ion. Therefore, the chemical formula for iron(II) oxide is FeO.
Question
Phosphorus tribromide (\({\text{PB}}{{\text{r}}_{\text{3}}}\)) is used to manufacture alprazolam, a drug used to treat anxiety disorders. Methanal (HCHO) is used as a disinfectant.
Consider the following reaction sequence:
![]()
a. (i) Deduce the balanced chemical equation for the reaction between sodium and sulfur. (ii)State the electron arrangements of the reactants and product, and (iii) explain whether sulfur is oxidized or reduced.[4]
b.Describe the acid-base character of the oxides of the period 3 elements, (i) Na (ii) Mg (iii) Al (iv) Si (v) P (vi) S and (vii) Cl.
State the balanced chemical equations for the reaction of each oxide with water.[7]
c. For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO:
(i)deduce the Lewis structure.
(ii)predict the shape and bond angle.[6]
d.i.Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.[2].
d.iiState the name of A.[1]
d.iii.Describe the redox behaviour of chromium with reference to oxidation numbers in the conversion of B to C. [1]
d.iv.Define the term oxidizing agent.
d.v.identify the oxidizing agent in the following reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {{\text{I}}^ – }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ – {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\] [2]
▶️Answer/Explanation
Markscheme
a.i.
\({\text{2Na(s)}} + {\text{S(s)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{2Na(s)}} + \frac{1}{2}{{\text{S}}_2}{\text{(g)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{16Na(s)}} + {{\text{S}}_2}{\text{(s)}} \to {\text{8N}}{{\text{a}}_2}{\text{S(s)}}\);
Ignore state symbols.
a.ii.Na: 2, 8,1 and S: 2, 8, 6;
\({\text{N}}{{\text{a}}^ + }\): 2, 8 and \({{\text{S}}^{2 – }}\): 2,8,8;
a.iii. reduced since it has gained electrons / reduced since oxidation number has decreased;
Do not award mark if incorrect oxidation numbers are given.
i. ii. Na, Mg: basic
iii. Al: amphoteric
Do not accept amphiprotic.
iv, v, vi, vii. Si to Cl: acidic
Award [2] for all three listed sets correct, [1] for one or two listed sets correct.
Award [1] for stating oxides become more basic towards left/Na and more acidic towards right/Cl.
Do not penalize incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Allow P2O3(s) \( + \) 3H2O(l) \( \to \) 2H3PO4(aq).
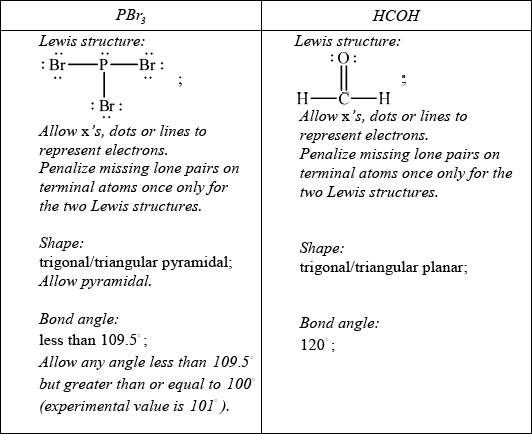
Do not allow ECF in this question from incorrect Lewis structure.
bond dipoles do not cancel / there is a net dipole / asymmetric distribution of electron cloud;
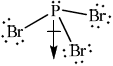
Allow polar bonds do not cancel or that it is an asymmetric molecule.
Award [2] for diagram showing net dipole moment as shown.
Do not award mark for chromium oxide.
d.iii.chromium is neither oxidized or reduced since there is no change in oxidation number/+6 to +6;
d.iv. substance reduced / causes other substance to be oxidized / increase oxidation number of another species / gains electrons / OWTTE;
d.v. Oxidizing agent:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }\) / dichromate (ion);
Detailed Solution:
a.
(i)
The reaction between sodium and sulfur can be represented by the following balanced chemical equation:
2 Na + S -> Na2S
In this equation, two atoms of sodium (2 Na) react with one molecule of sulfur (S) to form sodium sulfide (Na2S). The equation is balanced because the number of atoms on both sides of the equation is the same.
(ii) In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). When we write the configuration we’ll put all 11 electrons in orbitals around the nucleus of the Sodium atom. Therefore the sodium electron configuration will be 1s22s22p63s1.
In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we’ll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. Therefore the sulfur electron configuration will be 1s22s22p63s23p4.
Now, the sulfide anion, S2−, is formed when two electrons are added to a neutral sulfur atom.
Therefore the S2− electron configuration will be 1s22s22p63s23p6.
Na+ cation is formed when 1 electron is removed from neutral Na atom.
Therefore, the Na+ electron configuration will be 1s22s22p6
(iii) Here, S is reduced since it has gained electrons. Na is oxidized since it has lost electron.
b.
(i) Sodium Oxide (Na2O): Sodium oxide is a basic oxide. It reacts with water to form sodium hydroxide, a strong base.
The reaction can be represented as follows: Na2O + H2O -> 2NaOH
The presence of sodium hydroxide indicates the basic nature of sodium oxide.
(ii)Magnesium Oxide (MgO): Magnesium oxide is also a basic oxide. It reacts with water to form magnesium hydroxide, which is a weak base.
The reaction is as follows: MgO + H2O -> Mg(OH)2
The formation of magnesium hydroxide indicates the basic character of magnesium oxide.
(iii)Aluminum Oxide (Al2O3): Aluminum oxide is amphoteric, meaning it can exhibit both acidic and basic properties depending on the conditions. It reacts with strong bases to form aluminates, showing basic behavior, and with strong acids to form salts, exhibiting acidic behavior.
For example: Al2O3 + 6NaOH -> 2Na3AlO3 + 3H2O (basic behavior)
Al2O3 + 6HCl -> 2AlCl3 + 3H2O (acidic behavior)
(iv)Silicon Dioxide (SiO2): Silicon dioxide is a non-metallic oxide and is primarily acidic in nature.
It does not react with water to produce an acid or a base. Instead, it can react with strong bases or undergo hydrolysis to form silicic acid:
SiO2 + 2NaOH -> Na2SiO3 + H2O (basic behavior)
SiO2 + 2H2O -> H4SiO4 (silicic acid formation)
(v) Phosphorus Pentoxide (P2O5): Phosphorus pentoxide is an acidic oxide.
It reacts vigorously with water to form phosphoric acid: P2O5 + 3H2O -> 2H3PO4
The formation of phosphoric acid indicates the acidic nature of phosphorus pentoxide.
(vi) Sulfur Trioxide (SO3): Sulfur trioxide is also an acidic oxide.
It reacts with water to produce sulfuric acid: SO3 + H2O -> H2SO4
The formation of sulfuric acid confirms the acidic character of sulfur trioxide.
(vii) Chlorine Dioxide (Cl2O): Chlorine dioxide is an acidic oxide.
It reacts with water to produce chlorous acid: Cl2O + H2O -> 2HClO2
The formation of chlorous acid indicates the acidic nature of chlorine dioxide.
c.(i)
(ii) 
d.
(i) 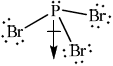
Phosphorus tribromide (PBr3) is a polar molecule due to the presence of a polar covalent bond and its molecular geometry.
PBr3 has a trigonal pyramidal molecular geometry, where the central phosphorus atom is bonded to three bromine atoms and has one lone pair of electrons. The three P-Br bonds are polar covalent because bromine is more electronegative than phosphorus. As a result, the bromine atoms exert a greater pull on the shared electrons, creating partial negative charges (δ-) on the bromine atoms and a partial positive charge (δ+) on the phosphorus atom.
Additionally, the presence of the lone pair of electrons on the phosphorus atom contributes to the overall polarity of the molecule. The lone pair of electrons exerts an additional electron density around the phosphorus atom, enhancing the polarization of the molecule.
(ii) A is Cr2O3 i.e. The chemical name for A is chromium(III) oxide.
(iii) In Cr2O3, 2Cr +3(-2) =0 i.e. Cr = +6
In Cr2O72- . -2=2Cr + 7(-2) i.e. Cr =+6
Hence, it is neither oxidized nor reduced.
(iv) An oxidizing agent, also known as an oxidant or oxidizer, is a substance that facilitates oxidation reactions by accepting electrons from another substance. In a redox (reduction-oxidation) reaction, the oxidizing agent itself undergoes reduction while causing the substance it reacts with to undergo oxidation.
(v) \[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {{\text{I}}^ – }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ – {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
Here, Cr2O72- is oxidizing agent as Cr is getting reduced from +6 to +3.
Question
1. Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
Define the terms
(i)Atomic number:
(ii)Mass number:
(iii)Isotopes of an element:
(iv)Distinguish between the terms group and period.
Deduce the electron arrangements of:
(v) \({\text{L}}{{\text{i}}^ + }\):
(vi) B:
(vii)Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for (viii) Li-6 and (ix) Li-7 isotope.
 [10]
[10]
Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the (ii) Ultraviolet and (iii) Visible regions of the spectrum. Label your diagram to show three transitions for each series.[6]
(iii) Iron is described as a transition metal. Identify the two most common ions of iron.
c.Deduce the chemical formulas of
(i)Lithium oxide:
(ii) Iron(II) oxide: [4]
▶️Answer/Explanation
Markscheme
(i) Atomic number:
number of protons (in nucleus/atom);
ii. Mass number:
(sum of) number of protons and neutrons (in nucleus/atom);
iii. Isotopes of an element:
atoms of same element / atoms with same number of protons/atomic number/Z but different number of neutrons/mass number/A;
Penalize once only use of the term element in the three definitions, for example, number of protons in an element or number of protons and neutrons in an element or element with the same atomic number but different mass number.
(iv) Group: (elements in vertical) columns in periodic table and Period: (elements in horizontal) rows in periodic table;
Allow elements in same group have similar chemical properties and within a period, atoms have same number of shells/energy levels (but number of electrons in valence/outer shell increases).
Allow groups distributed vertically and periods distributed horizontally / OWTTE.
Allow group number gives number of valence/outer shell electrons (for maingroup elements) and period gives same number of shells/energy levels.
(v) Li+: 2/1s2;
(vi) B: 2,3/1s22s22p1;
(vii) correct mathematical expression set-up \({\text{(e.g. }}\left( {\frac{x}{{100}}} \right)(10) + \left[ {\frac{{(100 – x)}}{{(100)}}} \right](11) = 10.81)\);
19%;
Award [2] for correct final answer.
(viii) 
Award [1 max] for correct number of neutrons for both isotopes if numbers of protons or electrons is not given.
Award [1 max] for correct number of protons and electrons for both isotopes if number of neutrons is not given or if numbers of neutrons are incorrect.
(i) Continuous spectrum: radiation spread over all wavelengths/frequencies/energies/colours / OWTTE;
Line spectrum: radiation (absorbed/emitted) at certain/specific wavelengths/frequencies/energies/colours / OWTTE;
Allow series of (separate/discrete) lines which converge/get closer together at high energy / OWTTE.
(ii) 
showing y-axis labelled as energy/E or labelling at least two energy levels
(\(n = 1\), \(n = 2\) etc. but not for \(n = 0\));
showing energy levels converging;
showing jumps to \(n = 1\) for ultraviolet series;
(iii) showing jumps to \(n = 2\) for visible series;
UV and visible must be labelled.
(i) metals have delocalized electrons / sea of electrons which are mobile/can move / OWTTE;
(ii)layers/positive ions/cations/atoms slide past/over each other / OWTTE;
Do not accept nuclei for M2.
(iii) Fe2+and Fe3+;
c.
(i) Lithium oxide: Li2O and
(ii) Iron(II) oxide: FeO;
Detailed Solution:
1.
(i) Atomic number: The atomic number of an element represents the number of protons found in the nucleus of an atom of that element. It is denoted by the symbol “Z” and is a unique characteristic of each element. The atomic number determines the identity of an element and its position on the periodic table.
(ii) Mass number: The mass number of an atom is the sum of the number of protons and neutrons present in the nucleus. It is denoted by the symbol “A”. The mass number provides an approximate measure of the total mass of an atom and is used to identify different isotopes of an element.
(iii) Isotopes: Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei. While isotopes have the same atomic number (number of protons) because they belong to the same element, they have different mass numbers due to the varying number of neutrons. Isotopes share similar chemical properties but may exhibit different physical properties, such as different atomic masses and radioactive behavior.
(iv) Group: A group, also known as a family, is a vertical column in the periodic table. Elements within the same group share similar chemical properties because they have the same number of valence electrons, which are the electrons in the outermost energy level of an atom. The group number indicates the number of valence electrons for elements in that group. For example, Group 1 elements, such as hydrogen (H) and sodium (Na), all have one valence electron.
Period: A period is a horizontal row in the periodic table. Elements in the same period do not necessarily share similar properties. Instead, the period number indicates the number of electron shells or energy levels occupied by the electrons in the atom. Each period represents a new energy level being filled with electrons.
(v)
The electronic arrangement of Li+ (lithium ion) can be determined by removing one electron from the neutral lithium atom (Li).
The electron configuration of a neutral lithium atom is 1s2 2s1.
By removing one electron, the electronic arrangement of Li+ becomes 1s2.
Since the lithium ion has lost one electron, it now has a filled 1s orbital and no valence electrons. The removal of the valence electron results in the formation of the lithium ion with a positive charge (Li+).
(vi) Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. Therefore the B electron configuration will be 1s22s22p1.
(vii) Relative atomic mass = Sum of product of isotope mass and fraction abundance
Relative atomic mass of Boron = 10.81
Let fraction of B-10 is x and B-11 is (1-x).
10.81 = 10x+11(1-x)
x=0.19
B-10 has 19% bundance.
(viii)
Lithium-6:
Number of protons: The atomic number of lithium is 3, which indicates that it has 3 protons.
Number of electrons: In a neutral atom, the number of electrons is equal to the number of protons. Therefore, lithium-6 also has 3 electrons.
Number of neutrons: To determine the number of neutrons, we subtract the atomic number (number of protons) from the mass number. For lithium-6, the mass number is 6, and since it has 3 protons, the number of neutrons would be 6 – 3 = 3.
So, lithium-6 has 3 protons, 3 electrons, and 3 neutrons.
(ix)
Lithium-7:
Number of protons: The atomic number of lithium is still 3, so lithium-7 also has 3 protons.
Number of electrons: Similar to lithium-6, lithium-7, as a neutral atom, has 3 electrons.
Number of neutrons: By subtracting the atomic number (3) from the mass number (7), we find that lithium-7 has 7 – 3 = 4 neutrons.
Thus, lithium-7 has 3 protons, 3 electrons, and 4 neutrons.
a.(i) A continuous spectrum and a line spectrum are two different types of spectra observed in the study of light emitted or absorbed by atoms or molecules. Here’s how they differ:
Continuous Spectrum: A continuous spectrum, also known as a continuous emission or absorption spectrum, is characterized by a continuous range of wavelengths or colors. It covers a broad range of wavelengths without any gaps or missing wavelengths. In a continuous spectrum, light is emitted or absorbed by a substance over a wide range of energies. An example of a continuous spectrum is the light emitted by an incandescent lamp or sunlight.
Line Spectrum: A line spectrum, also called a discrete spectrum or atomic spectrum, consists of only specific and distinct wavelengths or colors. It consists of individual lines or bands of light at specific wavelengths. Each line in a line spectrum corresponds to a particular energy transition within an atom or molecule. Line spectra are often observed when atoms or molecules emit or absorb light in a quantized manner. The most famous example is the emission spectrum of hydrogen, which consists of a series of discrete lines at specific wavelengths corresponding to different electron transitions within hydrogen atoms.
(ii) 
Lyman Series (Ultraviolet): The Lyman series corresponds to electron transitions to the n = 1 energy level. When an electron drops from higher energy levels to the n = 1 level, it emits photons in the ultraviolet region. The spectral lines in the Lyman series are not visible to the naked eye. The first line in the Lyman series (n = 2 to n = 1 transition) corresponds to the Lyman-alpha line at 121.6 nm.
(iii) Balmer Series (Visible): The Balmer series corresponds to electron transitions to the n = 2 energy level. When an electron drops from higher energy levels to the n = 2 level, it emits photons in the visible region of the electromagnetic spectrum. The Balmer series includes several visible spectral lines. The first line in the Balmer series (n = 3 to n = 2 transition) corresponds to the H-alpha line at 656.3 nm, which appears as red light.
b.
(i)Metals are good conductors of electricity due to the presence of delocalized electrons and their unique crystal structure. Here’s why metals exhibit high electrical conductivity: Delocalized electrons: In metallic bonding, outer electrons of metal atoms are loosely held and can move freely throughout the metal lattice. These electrons are not bound to a specific atom and are called delocalized electrons. They form an electron sea or cloud that can flow in response to an electric field. This mobility of delocalized electrons allows metals to conduct electricity efficiently.
(ii) Metals are malleable, meaning they can be easily hammered or bent into various shapes, due to the following factors:
Metallic bonding: In metals, the atoms are arranged in a closely-packed lattice structure. The positive metal ions are surrounded by a sea of delocalized electrons, which act as a binding force. The metallic bonds between positive ions and the shared electrons are strong, but not directional, allowing layers of atoms to slide past each other without breaking the bonds completely.
Plastic deformation: When an external force, such as hammering or pressure, is applied to a metal, the layers of metal ions can slide and shift relative to each other. This ability of metal atoms to move and retain their metallic bonding makes metals malleable. The delocalized electrons facilitate the movement of metal ions and maintain the integrity of the metal lattice.
(iii)Iron (Fe) is indeed classified as a transition metal. The two most common ions of iron are:
Iron(II) ion: The iron(II) ion is formed when iron loses two electrons. It has a +2 charge and is represented as Fe2+. This ion is also known as ferrous ion.
Iron(III) ion: The iron(III) ion is formed when iron loses three electrons. It has a +3 charge and is represented as Fe3+. This ion is also known as ferric ion.
c.
(i) Lithium oxide:
Lithium (Li) has a charge of +1, and oxygen (O) has a charge of -2. To balance the charges, we need two lithium ions to combine with one oxygen ion. Therefore, the chemical formula for lithium oxide is Li2O.
(ii) Iron(II) oxide:
Iron(II) (Fe2+) has a charge of +2, and oxygen (O) has a charge of -2. To balance the charges, we need one iron(II) ion to combine with one oxygen ion. Therefore, the chemical formula for iron(II) oxide is FeO.
