Question
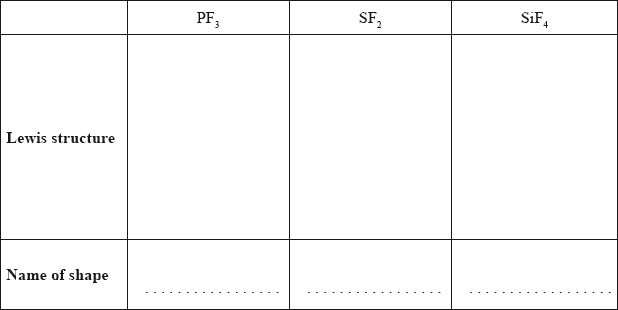
(i) PF3, (ii) SF2 and (iii) SiF4 have different shapes. Draw their Lewis structures and use the VSEPR theory to predict the name of the shape of each molecule.
▶️Answer/Explanation
Markscheme
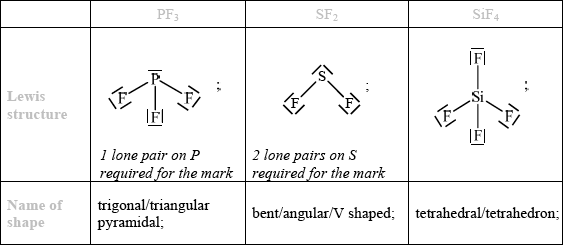
Penalise missing lone pairs on fluorine atoms once in correct structures only.
For Lewis structures candidates are not expected to draw exact shapes of molecules.
Do not allow ECF for wrong Lewis structures.
Accept dots or crosses instead of lines.
Detailed Solution:
(i) (ii) (iii)

PF3 : PF3 has a central phosphorus (P) atom bonded to three fluorine (F) atoms. The electron geometry around the central atom is tetrahedral, with the phosphorus atom at the center and the three fluorine atoms positioned around it. However, one of the positions is occupied by a lone pair of electrons on the phosphorus atom. The presence of the lone pair causes distortion of the molecular geometry. Therefore, PF3 has a trigonal pyramidal shape, with the three fluorine atoms arranged in a triangular shape around the phosphorus atom and the lone pair occupying the fourth position.
SF2 : SF2 has a central sulfur (S) atom bonded to two fluorine (F) atoms. The electron geometry around the central atom is trigonal planar, with the sulfur atom at the center and the two fluorine atoms positioned on opposite sides. However, one of the positions is occupied by a lone pair of electrons on the sulfur atom. The presence of the lone pair causes distortion of the molecular geometry. Therefore, SF2 has a bent or V-shaped shape, with the fluorine atoms arranged in a bent shape around the sulfur atom and the lone pair occupying one of the positions.
SiF4: SiF4 has a central silicon (Si) atom bonded to four fluorine (F) atoms. The electron geometry around the central atom is tetrahedral, with the silicon atom at the center and the four fluorine atoms positioned around it. The molecular geometry of SiF4 is also tetrahedral, with the fluorine atoms arranged symmetrically around the silicon atom.
Question
Alkenes are an economically and chemically important family of organic compounds.
a.i.The reaction of alkenes with bromine water provides a test for unsaturation in the laboratory. Describe the colour change when bromine water is added to chloroethene.
Deduce the Lewis structure of chloroethene and identify the formula of the repeating unit of the polymer poly(chloroethene).
(i) Deduce the structural formulas of the two alcohol isomers of molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Name each isomer and identify each as either a primary or a secondary alcohol.
Oxidation of the alcohol isomers lead to the formation of different organic products. Determine the structures of the organic products formed from the oxidation of the (ii)primary alcohol and the (iii)secondary alcohol isomer in (b) (i) above and list the conditions required to obtain the different products.
▶️Answer/Explanation
Markscheme
a.i.colour change from yellow/orange/rust colour/red/brown to colourless;
No mark for change to clear, or for decolourized with no reference to original colour.
Chloroethene:
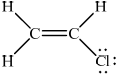 ;
;
No mark if the lone pairs missing on Cl.
Accept lines, dots or crosses for e– pairs.
Poly(chloroethene):
![]() ;
;
n and square brackets are not required.
Continuation bonds must be shown.
(i) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{OH}}\), propan-1-ol/1-propanol;
\({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_3}\), propan-2-ol/2-propanol;
Need both formula and name for mark.
Accept either condensed or full structural formulas.
CH3CH2CH2OH: primary and CH3CH(OH)CH3: secondary;
b(ii) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{CHO}}\);
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}}\);
\({\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}\);
Accept either condensed or full structural formulas.
from propan-1-ol: \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{CHO}}\) (propanal) obtained by distillation (as product is formed);
propan-1-ol gives \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}}\) (propanoic acid) by (heating under) reflux;
Award [1] if CH3CH2CHO and CH3CH2COOH identified but conditions not
given/incorrect.
biii. propan-2-ol gives \({\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}\) by (heating under) reflux;
Detailed Solution:
a.
(i) When bromine water is added to chloroethene, a rapid reaction takes place. The bromine molecule (Br2) undergoes an addition reaction with the double bond of chloroethene. This reaction is known as bromination. During the reaction, the reddish-brown color of bromine is quickly decolorized. The bromine molecule is broken and attached to the carbon atoms of the double bond in chloroethene, resulting in the formation of a colorless compound.
(ii) Chloroethene is : 
Chloroethene, also known as vinyl chloride, can undergo polymerization to form polyvinyl chloride (PVC). Polymerization is the process in which small monomer units are chemically linked together to form a larger polymer molecule. During polymerization, the double bond in chloroethene is opened, and the monomer units join together through a process called addition polymerization. ![]() .
.
b.
(i) The two structural isomers of C3H8O are:
Propanol (also known as n-propanol or 1-propanol): It has the molecular structure CH3CH2CH2OH. The hydroxyl (-OH) group is attached to the first carbon atom in the chain. It is a primary alcohol.
Isopropanol (also known as isopropyl alcohol or 2-propanol): It has the molecular structure (CH3)2CHOH. The hydroxyl group is attached to the second carbon atom, forming a branched structure. It is a secondary alcohol.
(ii) Propanol oxidizes as : \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{CHO}}\) (propanal) obtained by distillation.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}}\) (propanoic acid) by heating under reflux.
(iii) 2-propanol oxidizes as : \({\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}\) by heating under reflux.
