Question
Three compounds with similar relative molecular masses are butane, propanal and propan-1-ol.
List the three compounds in order of increasing boiling point (lowest first) and explain the differences in their boiling points.
Predict, with an explanation, which of the three compounds is least soluble or miscible in water.
When propan-1-ol is oxidized using a warm acidified solution of potassium dichromate(VI) two different organic products can be obtained. Deduce the name and structural formula for each of these two products.
Propan-2-ol is an isomer of propan-1-ol. Draw the structure of propan-2-ol.
Identify the class of alcohols that propan-2-ol belongs to and state the name of the organic product formed when it is oxidized by an acidified solution of potassium dichromate(VI).
▶️Answer/Explanation
Markscheme
butane \( < \) propanal\( < \) propan-1-ol;
butane has van der Waals/London/dispersion forces;
propanal has dipole-dipole attractive forces;
propan-1-ol has hydrogen bonding;
imf marks are independent of the order.
Treat references to bond breaking as contradictions if the imfs are correct.
butane is least soluble;
it cannot form hydrogen bonds/attractive forces with water molecules;
propanal and propanoic acid;
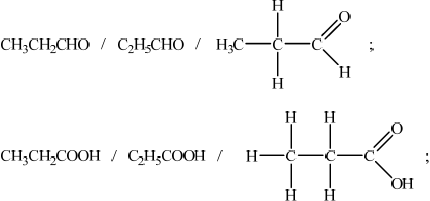
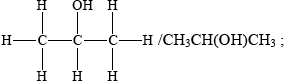
secondary (alcohol);
propanone / acetone;

Question
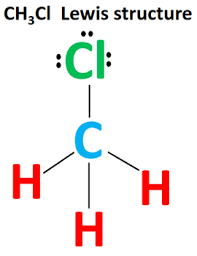
a.i.Draw the Lewis (electron dot) structure of chloromethane.
Predict the shape of the chloromethane molecule and the H–C–H bond angle.
Shape:
Bond angle:
Explain why chloromethane is a polar molecule.
Methanol has a lower molar mass than chloromethane. Explain why the boiling point of methanol is higher than that of chloromethane.
State the equation for the reaction between potassium and chlorine.
Outline the nature of the metallic bonding present in potassium.
Describe the covalent bond present in the chlorine molecule and how it is formed.
Describe the ionic bonding present in potassium chloride and how the ions are formed.
Potassium also reacts with water to form hydrogen gas. Determine the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of hydrogen gas that could theoretically be produced at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\) when 0.0587 g of potassium reacts with excess water.
Describe the acid-base character of the oxides of the period 3 elements, (i) Na (ii) Mg (iii) Al (iv) Si (v) P (vi) S and (vii) Cl.
State the balanced chemical equations for the reaction of each oxide with water.
▶️Answer/Explanation
Markscheme
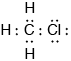 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
Shape: tetrahedral;
Bond angle: accept any value in the range: 108° to 111°;
(Literature value is 108.2°).
Cl is more electronegative than C / C–Cl bond polar;
bond dipoles do not cancel / asymmetric distribution of electron cloud / (resultant) net dipole moment (from vectorial addition of bond dipoles) going in direction of C–Cl axis / OWTTE;
hydrogen bonding in methanol;
stronger than dipole-dipole/van der Waals’ attractions/forces in chloromethane;
Accept converse argument.
a.v. \({\text{2K(s)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2KCl(s)}}\);
Ignore state symbols.
(electrostatic) attraction between lattice of cations/positive ions and delocalized electrons;
(electrostatic) attraction between positively charged nuclei and a pair of electrons;
formed as a result of electron sharing;
(electrostatic) attraction between positive and negative ions/oppositely charged ions/cations and anions;
formed as a result of transfer of an electron from a K atom to a Cl atom / OWTTE;
amount of potassium \( = \left( {\frac{{0.0587}}{{39.10}} = } \right){\text{ }}1.5 \times {10^{ – 3}}{\text{ (mol)}}\);
\({\text{2K}} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{2KO}} + {{\text{H}}_2}\) / amount of hydrogen \( = 7.50 \times {10^{ – 4}}{\text{ (mol)}}\);
volume of hydrogen \( = (7.50 \times {10^{ – 4}} \times 22.4 \times 1000 = ){\text{ }}16.8{\text{ (c}}{{\text{m}}^3}{\text{)}}\);
Accept calculation of volume of hydrogen using PV = nRT (answer is 16.9 cm3).
Award [3] for correct final answer.
Na, Mg (oxides): basic
Al (oxide): amphoteric
Do not accept amphiprotic.
Si to Cl (oxides): acidic
Award [2] for all three listed sets correct.
Award [1] for one or two listed sets correct.
Award [1] for stating oxides become more acidic towards right/Cl or more basic towards left/Na.
Do not penalize if reference is to Ar instead of Cl.
Do not penalize for incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Accept P2O5(s) + 3H2O(l) \( \to \) 2H3PO4(aq).
Do not award marks if incorrect formulas of the oxides are used.
Chloromethane (CH3Cl) is a polar molecule due to the difference in electronegativity between the carbon and chlorine atoms. Electronegativity is the ability of an atom to attract electrons towards itself in a covalent bond.
In CH3Cl, the chlorine atom is more electronegative than the carbon and hydrogen atoms. As a result, the chlorine atom pulls the shared electrons in the C-Cl bond closer to itself, creating a partial negative charge (δ-) on the chlorine atom and a partial positive charge (δ+) on the carbon atom.
Moreover, the three hydrogen atoms bonded to the carbon atom have a lower electronegativity than chlorine but higher than carbon. Although the electronegativity difference is not as significant as in the C-Cl bond, there is still some electron density being pulled towards the chlorine atom, creating a partial positive charge (δ+) on the hydrogen atoms. Overall, due to the polar bonds and the molecular geometry of CH3Cl (tetrahedral), the dipole moments do not cancel each other out, resulting in a net molecular dipole.
(iv) The boiling point of a compound is primarily determined by the strength of intermolecular forces between its molecules. In the case of methanol (CH3OH) and chloromethane (CH3Cl), methanol has a higher boiling point despite having a lower molar mass. This can be explained by considering the intermolecular forces present in each compound.
The polar nature of methanol enables it to form hydrogen bonds. Hydrogen bonding is a strong intermolecular force that occurs when a hydrogen atom bonded to an electronegative atom (such as oxygen) is attracted to the lone pair of electrons on another electronegative atom (such as oxygen or nitrogen) in a neighboring molecule. The hydrogen bonds in methanol are responsible for holding the molecules together in the liquid state.
In contrast, chloromethane has a smaller electronegativity difference between carbon and chlorine, resulting in a less polar molecule. Chloromethane can experience weaker dipole-dipole interactions due to the partial positive charge on the carbon atom and the partial negative charge on the chlorine atom. These dipole-dipole interactions are not as strong as hydrogen bonding in methanol.
(v)The reaction between potassium (K) and chlorine (Cl2) can be represented by the following equation:
2K + Cl2 → 2KCl
In this reaction, two potassium atoms combine with one chlorine molecule to form two molecules of potassium chloride.
b.
(i) The nature of metallic bonding in potassium involves the delocalization of valence electrons into an electron sea, the formation of positive metal cations, and the electrostatic attraction between the cations and the delocalized electrons. This bonding mechanism gives rise to the unique properties observed in metals.
(ii) In the chlorine molecule, each chlorine atom has seven valence electrons in its outermost energy level. Both chlorine atoms require one additional electron to achieve a stable octet (eight valence electrons) and attain a more stable electron configuration similar to the noble gas configuration of argon.
To achieve this stable electron configuration, the two chlorine atoms share a pair of electrons through overlapping orbitals. Each chlorine atom contributes one electron to form a single covalent bond. This shared electron pair fills the valence shell of each chlorine atom, resulting in a shared electron pair between the two atoms.
(iii) In potassium chloride, ionic bonding occurs through the transfer of an electron from the potassium atom to the chlorine atom. This transfer creates potassium cations (K+) and chloride anions (Cl-), which are then held together by strong electrostatic forces of attraction. This results in the formation of a crystal lattice structure in the solid state.
(iv)
Amount of potassium \( = \left( {\frac{{0.0587}}{{39.10}} = } \right){\text{ }}1.5 \times {10^{ – 3}}{\text{ (mol)}}\);
\({\text{2K}} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{2KO}} + {{\text{H}}_2}\) / amount of hydrogen \( = 7.50 \times {10^{ – 4}}{\text{ (mol)}}\);
volume of hydrogen \( = (7.50 \times {10^{ – 4}} \times 22.4 \times 1000 = ){\text{ }}16.8{\text{ (c}}{{\text{m}}^3}{\text{)}}\)
c.
(i) Sodium Oxide (Na2O): Sodium oxide is a basic oxide. It reacts with water to form sodium hydroxide, a strong base.
The reaction can be represented as follows: Na2O + H2O -> 2NaOH
The presence of sodium hydroxide indicates the basic nature of sodium oxide.
(ii)Magnesium Oxide (MgO): Magnesium oxide is also a basic oxide. It reacts with water to form magnesium hydroxide, which is a weak base.
The reaction is as follows: MgO + H2O -> Mg(OH)2
The formation of magnesium hydroxide indicates the basic character of magnesium oxide.
(iii)Aluminum Oxide (Al2O3): Aluminum oxide is amphoteric, meaning it can exhibit both acidic and basic properties depending on the conditions. It reacts with strong bases to form aluminates, showing basic behavior, and with strong acids to form salts, exhibiting acidic behavior.
For example: Al2O3 + 6NaOH -> 2Na3AlO3 + 3H2O (basic behavior)
Al2O3 + 6HCl -> 2AlCl3 + 3H2O (acidic behavior)
(iv)Silicon Dioxide (SiO2): Silicon dioxide is a non-metallic oxide and is primarily acidic in nature.
It does not react with water to produce an acid or a base. Instead, it can react with strong bases or undergo hydrolysis to form silicic acid:
SiO2 + 2NaOH -> Na2SiO3 + H2O (basic behavior)
SiO2 + 2H2O -> H4SiO4 (silicic acid formation)
(v) Phosphorus Pentoxide (P2O5): Phosphorus pentoxide is an acidic oxide.
It reacts vigorously with water to form phosphoric acid: P2O5 + 3H2O -> 2H3PO4
The formation of phosphoric acid indicates the acidic nature of phosphorus pentoxide.
(vi) Sulfur Trioxide (SO3): Sulfur trioxide is also an acidic oxide.
It reacts with water to produce sulfuric acid: SO3 + H2O -> H2SO4
The formation of sulfuric acid confirms the acidic character of sulfur trioxide.
(vii) Chlorine Dioxide (Cl2O): Chlorine dioxide is an acidic oxide.
It reacts with water to produce chlorous acid: Cl2O + H2O -> 2HClO2
The formation of chlorous acid indicates the acidic nature of chlorine dioxide.
Question
Three compounds with similar relative molecular masses are butane, propanal and propan-1-ol.
List the three compounds in order of increasing boiling point (lowest first) and explain the differences in their boiling points.
Predict, with an explanation, which of the three compounds is least soluble or miscible in water.
When propan-1-ol is oxidized using a warm acidified solution of potassium dichromate(VI) two different organic products can be obtained. Deduce the name and structural formula for each of these two products.
Propan-2-ol is an isomer of propan-1-ol. Draw the structure of propan-2-ol.
Identify the class of alcohols that propan-2-ol belongs to and state the name of the organic product formed when it is oxidized by an acidified solution of potassium dichromate(VI).
▶️Answer/Explanation
Markscheme
butane \( < \) propanal\( < \) propan-1-ol;
butane has van der Waals/London/dispersion forces;
propanal has dipole-dipole attractive forces;
propan-1-ol has hydrogen bonding;
imf marks are independent of the order.
Treat references to bond breaking as contradictions if the imfs are correct.
butane is least soluble;
it cannot form hydrogen bonds/attractive forces with water molecules;
propanal and propanoic acid;


secondary (alcohol);
propanone / acetone;

