Question
i. Define metallic bonding. [1]
ii. How metallic bonding contributes to electrical conductivity. [2]
▶️Answer/Explanation
Ans
i. electrostatic attraction between «a lattice of» cations/positive «metal» ions AND
ii. «a sea of» delocalized electrons mobile electrons responsible for conductivity OR
electrons move when a voltage/potential difference/electric field is applied
Do not accept “nuclei” for “cations/positive ions” in M2. Accept “mobile/free” for “delocalized” electrons in M2. Accept “electrons move when connected to a cell/battery/power supply” OR “electrons move when connected in a circuit” for M3.
Detailed Solution:
(i) Metallic bonding is defined as the sharing of delocalized electrons among positively charged metal cations. This results in the formation of a strong electrostatic attraction that holds the metal lattice together and imparts the characteristic properties of metals.
(ii) In metallic bonding, the valence electrons of metal atoms are not confined to individual atoms but are free to move throughout the metal lattice. These delocalized electrons form a “sea” of mobile charge carriers. Unlike in other types of bonding, such as covalent or ionic bonding, where electrons are localized between specific atoms, the delocalized electrons in metallic bonding are not bound to any particular metal ion. The delocalized electrons can move freely within the metal lattice. When an electric field is applied, the electrons can easily drift in response to the force exerted by the electric field. This mobility of electrons allows for the transmission of electrical charge throughout the metal.
Question
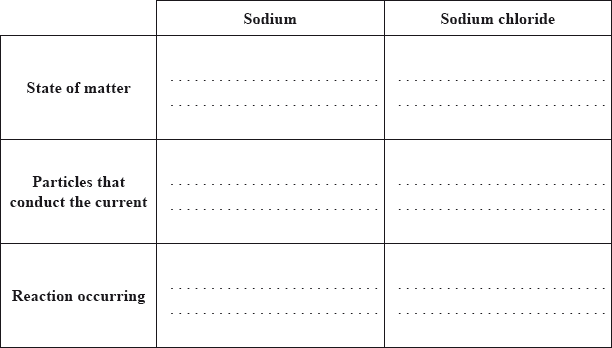
a. Both sodium and sodium chloride can conduct electricity.
Compare how electric current passes through (i) sodium and (ii) sodium chloride by completing the table below.
Sodium can be obtained by electrolysis from molten sodium chloride. Describe, using a diagram, the essential components of this electrolytic cell.
▶️Answer/Explanation
Markscheme
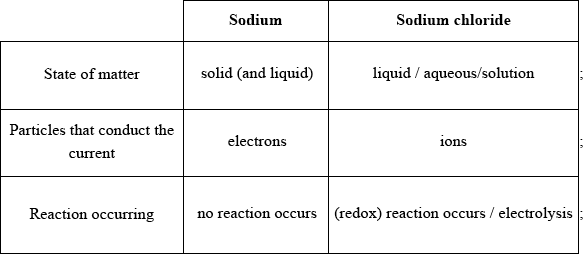
Award [1] for each feature that is correct for both sodium and sodium chloride.
Accept equation or half-equations for the reaction of sodium chloride in “reaction occurring”.
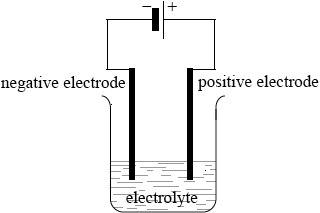
clear diagram containing all elements (power supply, connecting wires, electrodes, container and electrolyte);
labelled positive electrode/anode and negative electrode/cathode;
Accept positive and negative by correct symbols near power supply.
Accept power supply if shown as conventional long/short lines (as in diagram above) or clearly labelled DC power supply.
labelled electrolyte/NaCl(l);
State of NaCl not needed.

Electrolyte: The electrolyte is the molten sodium chloride, which serves as the medium for the flow of ions during the electrolysis process. Sodium chloride is heated to a high temperature (around 800-900°C) to melt it into a liquid state, breaking the ionic bond between sodium and chloride ions.
Electrodes: The electrolytic cell contains two electrodes, namely the anode and the cathode:
Anode: The anode is typically made of an inert material like graphite or platinum. It is connected to the positive terminal of the power supply. At the anode, chloride ions (Cl-) from the molten sodium chloride are oxidized, releasing electrons. The anode reaction involves the formation of chlorine gas (Cl2): 2Cl- → Cl2 + 2e-
Cathode: The cathode is also made of an inert material, often nickel or steel. It is connected to the negative terminal of the power supply. At the cathode, sodium ions (Na+) from the molten sodium chloride are reduced by gaining electrons. The cathode reaction involves the formation of sodium metal (Na): Na+ + e- → Na 3. Power Supply: A direct current (DC) power supply is required to provide a constant flow of electrons between the anode and cathode. It supplies electrical energy that drives the electrolysis process. The positive terminal of the power supply is connected to the anode, and the negative terminal is connected to the cathode.
4. Electrolytic Cell Container: The electrolytic cell is contained within a suitable container that can withstand high temperatures and chemical reactions. The container is often made of materials like steel or ceramic that are resistant to corrosion by the molten sodium chloride and other reaction products.

Question
a.i. A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
Phosphorus(V) oxide, \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{ }}({M_{\text{r}}} = 283.88)\), reacts vigorously with water \(({M_{\text{r}}} = 18.02)\), according to the equation below.
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{(s)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4}}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\]
Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
Describe the bonding in magnesium.
State an equation for the reaction of magnesium oxide with water.
A student added 5.00 g of \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) to 1.50 g of water. Determine the limiting reactant, showing your working.
Calculate the mass of phosphoric(V) acid, \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\), formed in the reaction in c.
State a balanced equation for the reaction of aqueous \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\) with excess aqueous sodium hydroxide, including state symbols.
State the formula of the conjugate base of \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\).
(i) Deduce the Lewis structure of \({\text{PH}}_4^ + \).
(ii) Predict, giving a reason, the bond angle around the phosphorus atom in \({\text{PH}}_4^ + \).
(iii) Predict whether or not the P–H bond is polar, giving a reason for your choice.
▶️Answer/Explanation
Markscheme
a.i. \(\left( {\frac{{(77.44 \times 24) + (10.00 \times 25) + (12.56{\text{ }}26)}}{{100}}} \right)\);
24.35;
Award [2] for correct final answer.
Two decimal places are required for M2.
Do not award any marks for 24.31 without showing method (as the value can be copied from the Data Booklet).
same atomic radii / 160 pm;
isotopes only differ by number of neutrons/size of nucleus / radius
determined by electron shells and number of protons / OWTTE;
Accept neutrons do not affect distance of electrons / OWTTE.
(lattice of) positive ions/cations and mobile/free/delocalized electrons;
Accept “sea of electrons” instead of “delocalized electrons”.
Award M1 for a suitable diagram.
electrostatic attraction (between ions and delocalized electrons);
\({\text{MgO}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_{\text{2}}}/{\text{M}}{{\text{g}}^{2 + }} + {\text{2O}}{{\text{H}}^ – }\);
Accept reversible arrow.
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{: }}\left( {\frac{{{\text{5.00}}}}{{{\text{283.88}}}} = } \right){\text{ 0.0176 (mol)}}\) and \({{\text{H}}_2}{\text{O: }}\left( {\frac{{{\text{1.50}}}}{{{\text{18.02}}}} = } \right){\text{ 0.0832 (mol)}}\);
\({{\text{H}}_2}{\text{O}}\) is the limiting reactant and reason related to stoichiometry;
\(\frac{{0.0832 \times 4}}{6}/0.0555{\text{ (mol)}}\);
\((0.0555 \times 98.00 = ){\text{ }}5.44{\text{ g}}\);
The unit is needed for M2.
Award [2] for correct final answer.
Do not penalize slight numerical variations due to premature rounding.
\({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3NaOH(aq)}} \to {\text{N}}{{\text{a}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\)
correct products and balancing;
correct state symbols;
Accept valid ionic equations.
\({{\text{H}}_2}{\text{PO}}_4^ – \);
(i)  ;
;
Accept dots, crosses or lines for pairs of electrons.
No need to distinguish the dative covalent bond from the other bonds.
Charge is required for the mark.
Do not penalize missing square brackets.
(ii) \(109^\circ 27’/109.5^\circ /109^\circ \);
4 electron domains/pairs/(negative) charge centres (around central atom/P);
Accept ion is tetrahedral / electron pairs/domains repel each other.
(iii) non-polar and P and H have the same electronegativity / OWTTE;
Accept slightly polar as precise electronegativities of P and H are not identical / OWTTE.
Detailed Solution:
a.
(i) Relative Atomic Mass = Sum of products of isotope mass and abundance
Relative Atomic Mass = 24*0.7744+25*0.10+26*0.1256
Relative Atomic Mass = 24.35
(ii) Isotopes of the same element have the same atomic radii. Isotopes have the same number of protons, which determines the atomic number and therefore the identity of the element. As a result, the atomic radii of magnesium isotopes 24Mg, 25Mg, and 26Mg would be essentially the same.
Therefore, the relative atomic radii of these magnesium isotopes would be considered equal.
(iii) Magnesium is a metallic element that forms metallic bonds. When multiple magnesium atoms come together, their cations form a lattice structure. In this structure, the cations are surrounded by a “sea” of delocalized valence electrons. The valence electrons are not associated with any particular magnesium ion but are free to move throughout the lattice.The delocalized valence electrons create a strong force of attraction between the positively charged magnesium cations and the negatively charged electron sea. This attraction, known as metallic bonding, holds the metal atoms together in a metallic crystal lattice.
b. Magnesium oxide reacts with water to form magnesium hydroxide.
\({\text{MgO}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_{\text{2}}}/{\text{M}}{{\text{g}}^{2 + }} + {\text{2O}}{{\text{H}}^ – }\)
c. Molar mass of P4O10 = 4*31 + 10*16 = 284 g
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{: }}\left( {\frac{{{\text{5.00}}}}{{{\text{284}}}} = } \right){\text{ 0.0176 (mol)}}\)
\({{\text{H}}_2}{\text{O: }}\left( {\frac{{{\text{1.50}}}}{{{\text{18}}}} = } \right){\text{ 0.0833 (mol)}}\)
P4O10 + 6 H2O -> 4 H3PO4
1 mole P4O10 requires 6 moles of H2O.
0.0176 moles P4O10 requires 0.1056 moles of H2O. We have only 0.0833 moles H2O.
Hence, H2O is the limiting reagent.
d.
(i) P4O10 + 6 H2O -> 4 H3PO4
6 moles H20 gives 4 moles of H3PO4
1 mole H2O gives 0.67 moles of H3PO4
0.0833 mole H2O gives 0.055 moles of H3PO4
Mass of H3PO4 produced = 0.055*98 = 5.39 g.
(ii)
The reaction between phosphoric acid (H3PO4) and sodium hydroxide (NaOH) is a neutralization reaction, resulting in the formation of sodium phosphate (Na3PO4) and water (H2O). Here’s the balanced chemical equation for the reaction:
H3PO4 + 3 NaOH -> Na3PO4 + 3 H2O.
(iii)
The conjugate base of phosphoric acid (H3PO4) is called dihydrogen phosphate ion or hydrogen phosphate ion (H2PO4-).
In the reaction of H3PO4 with a base, such as NaOH, H3PO4 donates a proton (H+) to the base, resulting in the formation of its conjugate base.
e.
(i) 
(ii)In the PH4+ ion, the phosphorus atom has a total of four bonding pairs of electrons. Since the hydrogen atom has only one valence electron, each hydrogen atom forms a single bond with the phosphorus atom. As a result, all four bonding pairs are concentrated around the central phosphorus atom.
The electron pair geometry around the central phosphorus atom in PH4+ is tetrahedral. This means that the four bonding pairs of electrons are arranged as far apart from each other as possible in three-dimensional space, forming a tetrahedron.
The bond angle in a tetrahedral arrangement is approximately 109.5 degrees. This is because the tetrahedral geometry maximizes the distance between the bonding pairs, minimizing electron-electron repulsion and achieving the most stable arrangement.
(iii) The electronegativity values of P and H are almost same. Electronegativity difference is almost zero. Hence, the P-H bond would be non polar.
