Question
There has been significant growth in the use of carbon nanotubes, CNT.
a. Explain these properties of carbon nanotubes.
b(i)CNT can act as Type 2 superconductors. Outline why Type 2 superconductors are generally more useful than Type 1.
b(iiExplain the role of electrons in superconducting materials in terms of the Bardeen-Cooper-Schrieffer (BCS) theory.
c(i)Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium-CNT alloy.
c(ii).Pre magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
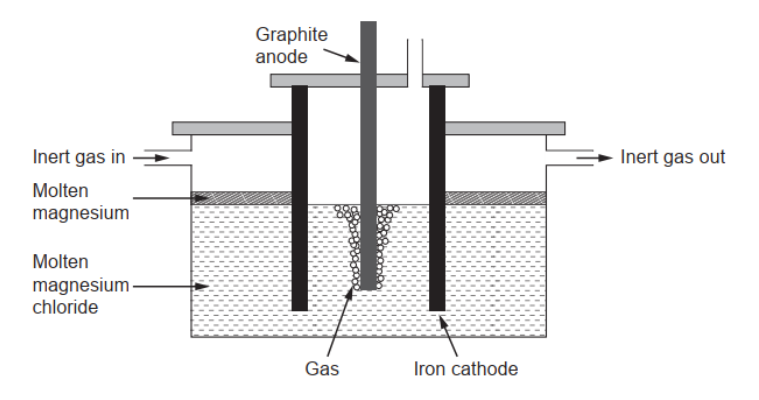
Calculate the theoretical mass of magnesium obtained if a current of $3.00 \mathrm{~A}$ is used for 10.0 hours. Use charge $:(Q)=$ current $(I) \times$ time $(t)$ and section 2 of the data booklet.
c(iiisuggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
d. Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
e. Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
▶️Answer/Explanation
Markscheme
a. Excellent strength: defect-free $\boldsymbol{A N D}$ rigid/regular 2D/3D
Excellent conductivity: delocalized electrons
Accept “carbons/atoms are all covalently bonded to each other” for M1.
$\mathrm{b}(\mathrm{i})$ Any two of:
have higher critical temperatures/ $T_{\mathrm{c}}$ «than Type 1 »
OR
can act at higher temperatures
have higher critical magnetic fields/ $B_{\mathrm{c}}$ «than Type $1 »$
less time needed to cool to operating temperature
less energy required to cool down/maintain low temperature
$\mathrm{b}(\mathrm{ii})$ Any three of:
passing electrons «slightly» deform lattice/displace positive ions/cations electrons couple/form Cooper pairs/condense with other electrons energy propagates along the lattice in wave-like manner/as phonons
Cooper pair/electron condensate/pair of electrons moves through lattice freely OR
phonons are «perfectly» elastic/cause no energy loss
c(i)Any of:
ductility
strength/resistance to deformation
malleability
hardness
resistance to corrosion/chemical resistance
range of working temperatures
density
Do not accept “conductivity”.
$$
\begin{gathered}
\mathrm{c}(\mathrm{ii}) \leqslant Q=I \times t=3.00 \times 10.0 \times 3600=» 108000 \mathrm{C} \\
« \frac{Q}{F}=\frac{108000 \mathrm{C}}{96500 \mathrm{C} \mathrm{mol}^{-1}}=» 1.12 \ll \mathrm{mol} \mathrm{e}^{-} » \\
« \frac{1.12 \mathrm{~mol}}{2}=0.560 \mathrm{~mol} \mathrm{Mg} » \\
« m=0.560 \mathrm{~mol} \times 24.31 \mathrm{~g} \mathrm{~mol}^{-1}=» 13.6 《 \mathrm{~g} »
\end{gathered}
$$
c(iiiłrgon/Ar/helium/He
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen $/ \mathrm{N}_2$ “.
d. pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react
Accept “molecules/ions” for “reactants” in M2.
e. rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order AND have «some» directional order/pattern
Question
Aluminium and iron are both widely used in modern society.
Almost all iron is used in the form of an alloy. State the name of the most common type of iron alloy and the other element that is an essential component of these alloys.
Name:
Other element:
An early alloy of aluminium was Duralumin which contained small quantities of copper and magnesium. This is stronger and more rigid than pure aluminium. Explain on an atomic level why the addition of other elements has this effect.
▶️Answer/Explanation
Markscheme
Name: steel and other element: carbon;
atoms/ions of the alloying element are a different size/larger/smaller;
prevents the layers of atoms/ions sliding across each other;
Examiners report
This question was generally answered correctly.
Part (d) proved to be most challenging although it deals with the fundamental idea of the behaviour of alloys.
Question
Steel is an alloy of iron, carbon and other metallic and non-metallic elements. Stainless steel contains about 18% chromium and 8% nickel. Explain why iron can form alloys with other transition metals.
▶️Answer/Explanation
Markscheme
their atoms/ions have similar/slightly different radii/size/densities/properties;
so do not disrupt crystal structure/metallic lattice significantly / OWTTE;
Examiners report
Part (b) was very poorly answered with most candidates providing general explanations of the formation of alloys.

