Question
Red supergiant stars contain carbon- 12 formed by the fusion of helium- 4 nuclei with beryllium- 8 nuclei.
Mass of a helium- 4 nucleus $=4.002602 \mathrm{amu}$
Mass of a beryllium -8 nucleus $=8.005305 \mathrm{amu}$
Mass of a carbon -12 nucleus $=12.000000 \mathrm{amu}$
a(i)State the nuclear equation for the fusion reaction.
a(iiExplain why fusion is an exothermic process.
b. Beryllium-8 is a radioactive isotope with a half-life of $6.70 \times 10^{-17} \mathrm{~s}$.
Calculate the mass of beryllium-8 remaining after $2.01 \times 10^{-16}$ s from a sample initially containing $4.00 \mathrm{~g}$ of beryllium-8.
▶️Answer/Explanation
Markscheme
$$
\mathrm{a}(\mathrm{i}){ }_2^4 \mathrm{He}+{ }_4^8 \mathrm{Be} \rightarrow{ }_6^{12} \mathrm{C}
$$
NOTE: Do not penalize missing atomic numbers.
a(ii)ALTERNATIVE 1
binding energy per nucleon is larger in carbon-12/product «than beryllium- 8 and helium-4/reactants»
difference in «total» binding energy is released «during fusion»
ALTERNATIVE 2
mass of carbon-12/product «nucleus» is less than «the sum of» the masses of helium-4 and beryllium- 8 «nuclei»/reactants $\boldsymbol{O R}$
two smaller nuclei form a lager nucleus
mass lost/difference is converted to energy «and released»
$\boldsymbol{O R}$
$E=m c^2$
b. ALTERNATIVE 1
3 half-lives $\checkmark$
$0.500 \mathrm{~g}$ «of beryllium-8 remain» $\checkmark$
ALTERNATIVE 2
$\mathrm{m}=4.00\left(\frac{1}{2}\right)^{\frac{2.01 \times 10^{-16}}{6.70 \times 10^{-17}}}$
$0.500 \mathrm{~g}$ «of beryllium-8 remain» $\checkmark$
ALTERNATIVE 3
$$
\begin{aligned}
& \lambda=《 \frac{\ln 2}{6.70 \times 10^{-17}} \gg=1.03 \times 10^{16}\left\langle\mathrm{~s}^{-1} \gg\right. \\
& m=《 4.00 \mathrm{e}^{-1.03 \times 10^{16} \times 2.01 \times 10^{-16}}=\gg 0.500 \ll \mathrm{g} \gg
\end{aligned}
$$
Question
\text { Powdered zinc was reacted with } 25.00 \mathrm{~cm}^3 \text { of } 1.000 \mathrm{~mol} \mathrm{dm}{ }^{-3} \text { copper(II) sulfate solution in an insulated beaker. Temperature was plotted against time. }
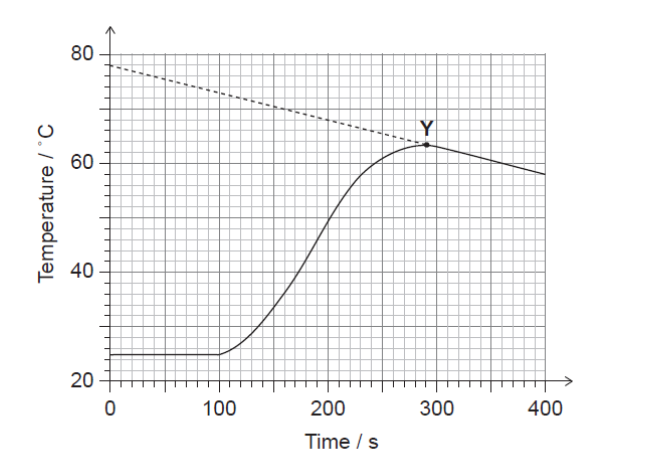
a(i)Estimate the time at which the powdered zinc was placed in the beaker.
a(iißState what point $\mathbf{Y}$ on the graph represents.
b(i).The maximum temperature used to calculate the enthalpy of reaction was chosen at a point on the extrapolated (dotted) line.
State the maximum temperature which should be used and outline one assumption made in choosing this temperature on the extrapolated line.
Maximum temperature:
Assumption:
b(ii)To determine the enthalpy of reaction the experiment was carried out five times. The same volume and concentration of copper(II) sulfate was used but the mass of zinc was different each time. Suggest, with a reason, if zinc or copper(II) sulfate should be in excess for each trial.
$\mathrm{b}\left(\right.$ iiiT), formula $q=m c \Delta T$ was used to calculate the energy released. The values used in the calculation were $m=25.00 \mathrm{~g}, c=4.18 \mathrm{~J} \mathrm{~g}^{-1} \mathrm{~K}^{-1}$.
State an assumption made when using these values for $m$ and $c$.
b(iv).If.redict, giving a reason, how the final enthalpy of reaction calculated from this experiment would compare with the theoretical value.
▶️Answer/Explanation
Markscheme
$a(i) 100 « s » \quad[/]$
Note: Accept 90 to $100 \mathrm{~s}$.
a(ii)ighest recorded temperature
OR
when rate of heat production equals rate of heat loss [ []
b(i)Maximum temperature:
$73 «^{\circ} \mathrm{C} » \quad[/]$
Assumption:
«temperature reached if» reaction instantaneous
OR
«temperature reached if reaction occurred» without heat loss [ [ ]
Note: Accept “rate of heat loss is constant” OR “rate of temperature decrease is constant”.
b(ii)Any one of:
copper(II) sulfate AND mass/amount of zinc is independent variable/being changed
OR
copper(II) sulfate AND with zinc in excess there is no independent variable «as amount of copper(II) sulfate is fixed» [ $\boldsymbol{V}$ ]
copper(II) sulfate AND having excess zinc will not yield different results in each trial [ $\boldsymbol{V}]$
zinc $A N D$ results can be used to see if amount of zinc affects temperature rise «so this can be allowed for» [ $\boldsymbol{\sim}]$
zinc $A N D$ reduces variables/keeps the amount reacting constant $\quad[\boldsymbol{\sim}]$
b.(iii)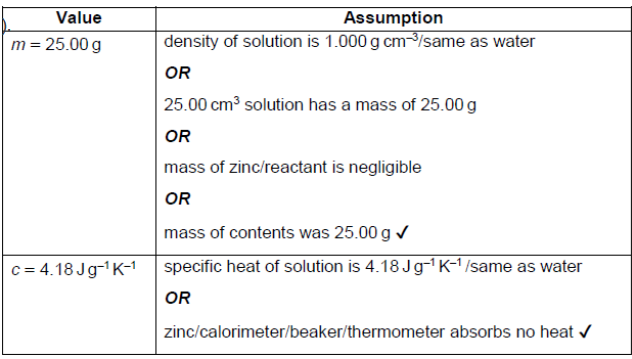
b(iv)lower/less exothermic/less negative AND heat loss/some heat not accounted for OR
lower/less exothermic/less negative AND mass of reaction mixture greater than $25.00 \mathrm{~g}$ OR
greater/more exothermic/more negative AND specific heat of solution less than water $[\boldsymbol{V}]$
Question
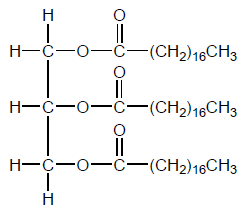
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
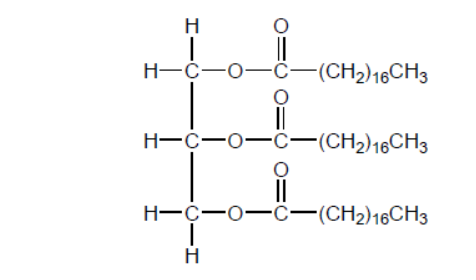
a. State two reagents required to convert vegetable oil to biodiesel.
b. Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
c. Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
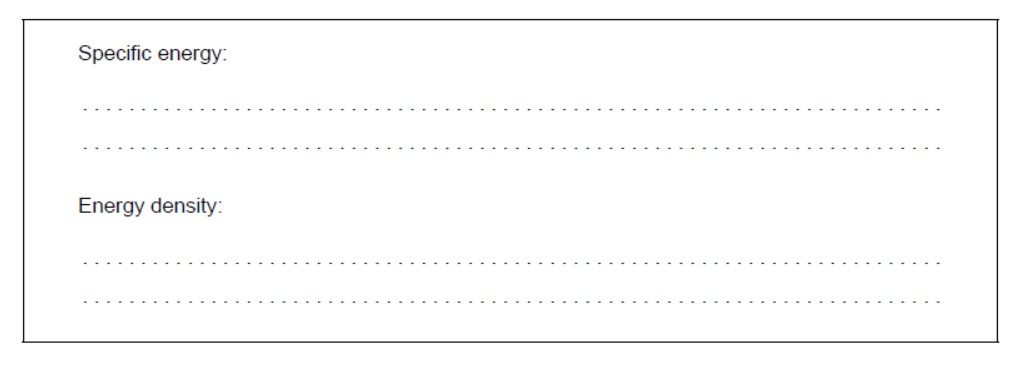
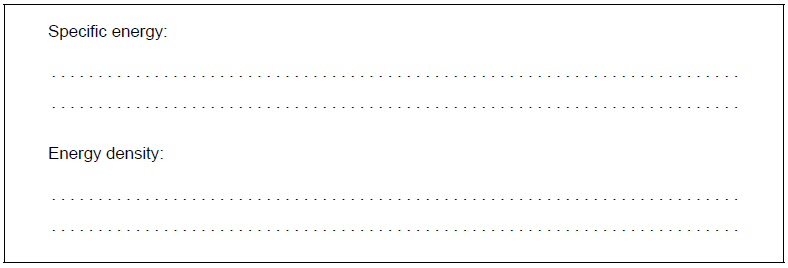
d. Determine the specific energy, in $\mathrm{kJ} \mathrm{g}^{-1}$, and energy density, in $\mathrm{kJ} \mathrm{cm}^{-3}$, of a particular biodiesel using the following data and section 1 of the data booklet.
Density $=0.850 \mathrm{~g} \mathrm{~cm}^{-3}$; Molar mass $=299 \mathrm{~g} \mathrm{~mol}^{-1}$;
Enthalpy of combustion $=12.0 \mathrm{MJ} \mathrm{mol}^{-1}$.
▶️Answer/Explanation
Markscheme
a. methanol
OR
ethanol
strong acid
OR
strong base
Accept “alcohol”.
Accept any specific strong acid or strong base other than $\mathrm{HNO}_3$ /nitric acid.
[3 marks]
b. $\mathrm{CH}_3\left(\mathrm{CH}_2\right)_{16} \mathrm{COOCH}_3 / \mathrm{CH}_3 \mathrm{OCO}\left(\mathrm{CH}_2\right){ }_{16} \mathrm{CH}_3$
OR
$$
\mathrm{CH}_3\left(\mathrm{CH}_2\right)_{16} \mathrm{COOC}_2 \mathrm{H}_5 / \mathrm{C}_2 \mathrm{H}_5 \mathrm{OCO}\left(\mathrm{CH}_2\right)_{16} \mathrm{CH}_3
$$
Product must correspond to alcohol chosen in (a), but award mark for either structure if neither given for (a).
weaker intermolecular/dispersion/London/van der Waals’ forces OR
smaller/shorter molecules
Accept “lower molecular mass/ $M_r$ ” or “lower number of electrons”.
Accept converse arguments.
[2 marks]
d. Specific energy: «= $\frac{12000 \mathrm{~kJ} \mathrm{~mol}^{-1}}{299 \mathrm{~g} \mathrm{~mol}^{-1}} »=40.1 « \mathrm{~kJ} \mathrm{~g}^{-1} »$
Energy density: « $=40.1 \mathrm{~kJ} \mathrm{~g}^{-1} \times 0.850 \mathrm{~g} \mathrm{~cm}^{-3} »=34.1 « \mathrm{~kJ} \mathrm{~cm}^{-3}$ »
Award [1] if both are in terms of a unit other than $\mathrm{kJ}$ (such as $\mathrm{J}$ or MJ).
Question
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution.
The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a $\mathrm{pH}$ probe. Their results are given below.
Volume of aqueous $\mathrm{NaOH}=25.0 \pm 0.5 \mathrm{~cm}^3$
Concentration of $\mathrm{HCl}=1.00 \pm 0.01 \mathrm{~mol} \mathrm{dm}^{-3}$
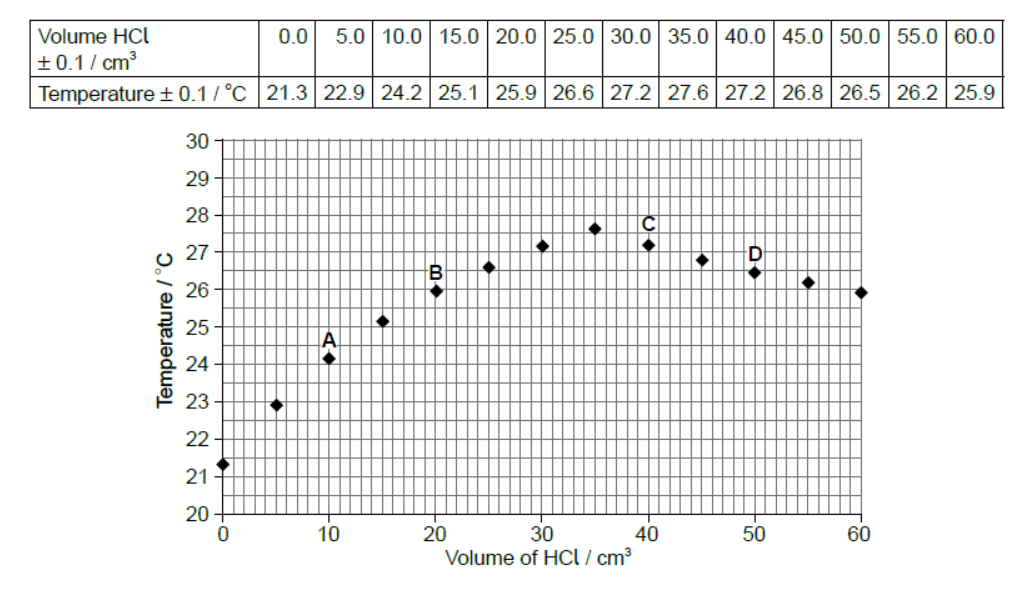
The graph of temperature against titre can be used to calculate the concentration of alkali without knowing the concentration of the hydrochloric acid, using the enthalpy of neutralization.
a. Explain how the concentration may be calculated in this way.
[2]
b. Heat losses would make this method less accurate than the $\mathrm{pH}$ probe method. Outline why the thermometric method would always give a lower,
[2] not a higher, concentration.
c. Suggest how heat loss could be reduced.
[1]
d. State one other assumption that is usually made in the calculation of the heat produced.
[1]
e. Suggest why scientists often make assumptions that do not correspond to reality.
f. Outline why the thermochemical method would not be appropriate for $0.001 \mathrm{~mol} \mathrm{dm}^{-3}$ hydrochloric acid and aqueous sodium hydroxide of a
similar concentration.
▶️Answer/Explanation
Markscheme
a. heat change/evolved can be calculated from the «maximum» temperature increase and the mass of solution
OR
$$
q=m c \Delta T
$$
heat «evolved» gives the number of moles «of both acid and alkali present when neutralisation occurs»
OR
$$
\mathrm{n}=\frac{\mathrm{q}}{\Delta \mathrm{H}_{\text {neut }}}
$$
volume «of acid and the volume of alkali required to just neutralise each other» can be used to calculate the concentration«s of both» OR
$$
[\mathrm{NaOH}]=\frac{\mathrm{n}}{\mathrm{V}}
$$
[2 marks]
b. smaller temperature increase/ $\Delta T$
OR
heat released would «appear to» be less
amount of substance/n calculated is smaller
c. using «expanded» polystyrene cup
OR
insulating beaker
OR
putting a lid on beaker
Do not accept calorimeter by itself.
Accept any other reasonable suggestion.
[1 mark]
d. “specific» heat capacity of the beaker/container/thermometer is ignored
OR
density of the solutions is assumed as $1.00 \mathrm{~g} \mathrm{~cm}^{-3} / \mathrm{same}$ as water
OR
specific heat capacity of the solutions is assumed as $4.18 \mathrm{~J} \mathrm{~g}^{-1} \mathrm{~K}^{-1} /$ same as water
Accept “reaction goes to completion”.
Accept “reaction is conducted under standard conditions”.
Accept “no evaporation occurs”.
Accept any other relevant valid assumption.
Do not accept “heat is not released from other reactions”.
[1 mark]
e. allows simple theories to be applied to real life situations
OR
enables us to start to understand complex situations
OR
gives answers that are accurate to the required order of magnitude
OR
simplifies the calculations involved
Do not accept “to simplify the situation” without further detail.
Accept “errors do not have a major impact on the results”.
[1 mark]
f. temperature rise would be too small «to be accurately measured»
Accept “heat released would be too small “to be accurately measured»”.
Question
Granola bars are a source of dietary fibre.
When 1.13 g of a granola bar was combusted in a bomb calorimeter, the temperature of \({\text{500 c}}{{\text{m}}^{\text{3}}}\) of water increased from 18.5 °C to 28.0 °C. Calculate the energy value, in kJ per 100 g, of the granola bar to the correct number of significant figures.
▶️Answer/Explanation
Markscheme
energy (released by granola) \( = (500 \times 4.18 \times 9.5 = ){\text{ }}19855{\text{ (J)}}/2.0 \times {10^4}{\text{ (J)}}\);
energy released (in kJ per 100 g) \(\left( {\frac{{19855}}{{1000}} \times \frac{{100}}{{1.13}} = } \right){\text{ }}1757.079\);
\( = 1.8 \times {10^3}{\text{ (kJ per 100 g)}}\);
Allow 1800 (kJ per 100 g).
Award [3] for correct final answer.
Award [2 max] for 1760 (kJ per 100 g).
Remember to allow ECF.
Examiners report
Candidates generally used the appropriate equations to solve for the energy value for part (a) however, many included 1.13g with the mass of water and on quite a few occasions, \({\text{22.4 d}}{{\text{m}}^{\text{3}}}\) was used in the question for to calculate energy per gram. Several missed the final mark for not expressing the final value in 2 significant figures. Candidates were not able to apply the rules for addition and subtraction within the mathematical process. Surprisingly defining dietary fibre in part (b) scored poorly. Many omitted reference to plant material or cellulose; there appears to be a general misunderstanding that any food that is not digested is dietary fibre. Most candidates were able to name two health problems for (b)(ii).
Question
Glucose, C6H12O6, is a monosaccharide that our body can use as a source of energy.
Deduce the equation for the cellular respiration of glucose.
Calculate the energy, in kJ, produced from 15.0g of glucose if its enthalpy of combustion is −2803kJmol−1.
Glucose is the basic building block of starch which can be used to make bioplastics. Outline two advantages and two disadvantages of biodegradable plastics.
Two advantages:
Two disadvantages:
Bioplastics are broken down by enzyme catalysed reactions. Sketch a graph illustrating how the rate of this reaction varies with pH.
▶️Answer/Explanation
Markscheme
C6H12O6 (aq) + 6O2 (aq) → 6CO2 (aq) + 6H2O (l)
Accept equations for anaerobic respiration, such as C6H12O6 (aq) → 2C3H6O3 (aq)
Ignore ATP if added as a product.
\(n\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}} \right)\left\langle { = \frac{{15.0}}{{180.18}}} \right\rangle = 0.0833 \ll {\rm{mol}} \gg \)
«energy=0.0833×2803=»233«kJ»
Award [2] for correct final answer.
Accept -233«kJ».
Two advantages:
renewable resource
broken down/digested by bacteria or other organisms within a relatively short time/quickly
reduce «volume of» plastic waste/landfill
reduce use of petrochemicals
OR
reduce use of fossil fuels as hydrocarbon source
degrade into non-toxic products
Any two advantages for [2 max].
M2: reference must be made to time. Do not accept “biodegradable” (since stated in question).
Ignore any mention of cost.
Two disadvantages:
require use of land «for crop production»
increased use of fertilizers/pesticides «leading to pollution»
OR
eutrophication
might break down before end of use
release of methane/CH4/greenhouse gas «during degradation»
Any two disadvantages for [2 max].
Ignore any mention of cost.
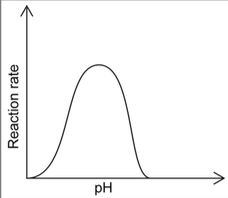
typical curve as shown in example above √
Accept any curve with a single maximum (not just bell-shaped).
Ignore features such as pH values on a pH scale or a pH value at maximum (if given).
Do not penalize if curve does not touch the x-axis.
Question
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
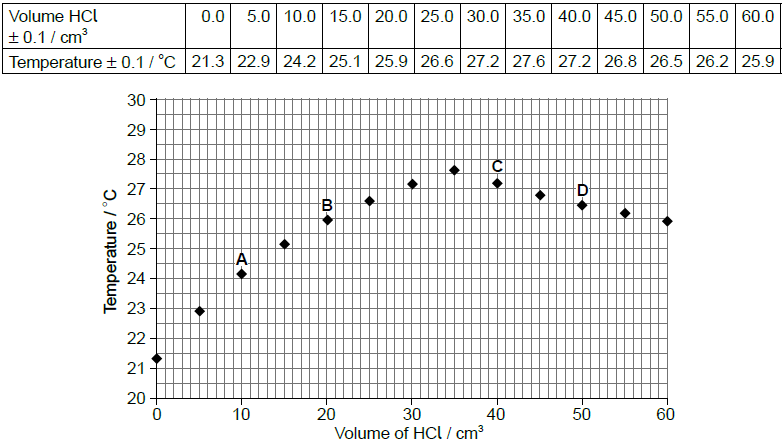
The graph of temperature against titre can be used to calculate the concentration of alkali without knowing the concentration of the hydrochloric acid, using the enthalpy of neutralization.
Explain how the concentration may be calculated in this way.
Heat losses would make this method less accurate than the pH probe method. Outline why the thermometric method would always give a lower, not a higher, concentration.
Suggest how heat loss could be reduced.
State one other assumption that is usually made in the calculation of the heat produced.
Suggest why scientists often make assumptions that do not correspond to reality.
Outline why the thermochemical method would not be appropriate for 0.001 mol\(\,\)dm−3 hydrochloric acid and aqueous sodium hydroxide of a similar concentration.
▶️Answer/Explanation
Markscheme
heat change/evolved can be calculated from the «maximum» temperature increase and the mass of solution
OR
q = mcΔT
heat «evolved» gives the number of moles «of both acid and alkali present when neutralisation occurs»
OR
\(n = \frac{q}{{\Delta {H_{neut}}}}\)
volume «of acid and the volume of alkali required to just neutralise each other» can be used to calculate the concentration«s of both»
OR
\(\left[ {{\text{NaOH}}} \right] = \frac{n}{V}\)
[2 marks]
smaller temperature increase/ΔT
OR
heat released would «appear to» be less
amount of substance/n calculated is smaller
[2 marks]
using «expanded» polystyrene cup
OR
insulating beaker
OR
putting a lid on beaker
Do not accept calorimeter by itself.
Accept any other reasonable suggestion.
[1 mark]
«specific» heat capacity of the beaker/container/thermometer is ignored
OR
density of the solutions is assumed as 1.00 g\(\,\)cm–3/same as water
OR
specific heat capacity of the solutions is assumed as 4.18 J g–1\(\,\)K–1/same as water
Accept “reaction goes to completion”.
Accept “reaction is conducted under standard conditions”.
Accept “no evaporation occurs”.
Accept any other relevant valid assumption.
Do not accept “heat is not released from other reactions”.
[1 mark]
allows simple theories to be applied to real life situations
OR
enables us to start to understand complex situations
OR
gives answers that are accurate to the required order of magnitude
OR
simplifies the calculations involved
Do not accept “to simplify the situation” without further detail.
Accept “errors do not have a major impact on the results”.
[1 mark]
temperature rise would be too small «to be accurately measured»
Accept “heat released would be too small «to be accurately measured»”.
[1 mark]
Question
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
State two reagents required to convert vegetable oil to biodiesel.
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
Determine the specific energy, in kJ\(\,\)g−1, and energy density, in kJ\(\,\)cm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 g\(\,\)cm−3; Molar mass = 299 g\(\,\)mol−1;
Enthalpy of combustion = 12.0 MJ\(\,\)mol−1.
▶️Answer/Explanation
Markscheme
methanol
OR
ethanol
strong acid
OR
strong base
Accept “alcohol”.
Accept any specific strong acid or strong base other than HNO3/nitric acid.
[3 marks]
CH3(CH2)16COOCH3 / CH3OCO(CH2)16CH3
OR
CH3(CH2)16COOC2H5 / C2H5OCO(CH2)16CH3
Product must correspond to alcohol chosen in (a), but award mark for either structure if neither given for (a).
[1 mark]
lower viscosity
weaker intermolecular/dispersion/London/van der Waals’ forces
OR
smaller/shorter molecules
Accept “lower molecular mass/Mr” or “lower number of electrons”.
Accept converse arguments.
[2 marks]
Specific energy: «\( = \frac{{12\,000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}{{299{\text{ g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)» = 40.1 «kJ g−1»
Energy density: «= 40.1 kJ\(\,\)g−1 x 0.850 g\(\,\)cm−3» = 34.1 «kJ\(\,\)cm−3»
Award [1] if both are in terms of a unit other than kJ (such as J or MJ).
[2 marks]

