Question
The potential energy profile of a reaction is shown.
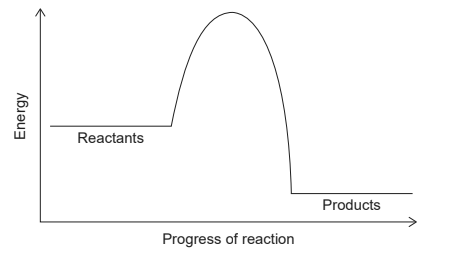
What can be determined about stability and energy change from the potential energy profile shown?
| More stable | Reaction |
A | reactants | exothermic |
B | reactants | endothermic |
C | products | exothermic |
D | products | endothermic |
▶️Answer/Explanation
C
Here, the products have a smaller quantity of energy than the reactants, which means energy is released during the reaction. Hence, the reaction is an exothermic reaction and the products are more stable than reactants.
Question
Which equation represents the bond enthalpy for the H–Br bond in hydrogen bromide?
A. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(g)}}\)
B. \({\text{HBr(g)}} \to {\text{H(g)}} + {\text{Br(l)}}\)
C. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{l)}}\)
D. \({\text{HBr(g)}} \to {\text{H(g)}} + \frac{1}{2}{\text{B}}{{\text{r}}_2}({\text{g)}}\)
▶️Answer/Explanation
A
The bond dissociation energy is the energy required—an endothermic process—to break a bond and form two atomic or molecular fragments, each with one electron of the original shared pair. A represents the bond enthalpy for the H–Br bond in hydrogen bromide.
Question
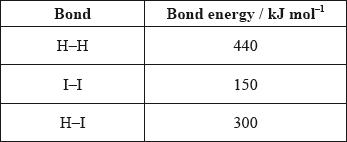
Use the average bond enthalpies below to calculate the enthalpy change, in kJ, for the following reaction.
\[{{\text{H}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}} \to {\text{2HI(g)}}\]
A. +290
B. +10
C. –10
D. –290
▶️Answer/Explanation
C
\[{{\text{H}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}} \to {\text{2HI(g)}}\]
ΔHrxn= Energy required to break the bond + Energy released in the formation of a new bond
Here, 1 H-H and 1 I-I bond is broken and 2 H-I bonds are formed.
ΔHrxn = (H-H bond energy + I-I bond energy) – 2(H-I bond energy)
= 440+150-2(300)
= -10 kJ
Question
Which processes are exothermic?
I. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2Cl(g)}}\)
III. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(aq)}} + {\text{NaOH(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COONa(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
A is combustion, so it is exothermic.
C is acid base neutralization. In a neutralization reaction, an acid and a base react to form salt and water. Also, it’s important to understand that during an exothermic reaction, bonds are being made and energy is released to the surroundings. This is what ultimately happens during a neutralization reaction that gives its exothermic character.
In B, the reaction is endothermic as it takes energy to break the Cl2 bond.
