Question
0.100 g of magnesium ribbon is added to \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) sulfuric acid to produce hydrogen gas and magnesium sulfate.
\[{\text{Mg(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {{\text{H}}_2}{\text{(g)}} + {\text{MgS}}{{\text{O}}_4}{\text{(aq)}}\]
Magnesium sulfate can exist in either the hydrated form or in the anhydrous form. Two students wished to determine the enthalpy of hydration of anhydrous magnesium sulfate. They measured the initial and the highest temperature reached when anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}{\text{(s)}}\), was dissolved in water. They presented their results in the following table.

The students repeated the experiment using 6.16 g of solid hydrated magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}} \bullet {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(s)}}\), and \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. They found the enthalpy change, \(\Delta {H_2}\), to be \( + 18{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\).
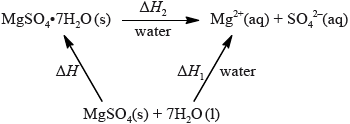
The enthalpy of hydration of solid anhydrous magnesium sulfate is difficult to determine experimentally, but can be determined using the diagram below.
Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic limestone. This limestone is a mixture of magnesium carbonate and calcium carbonate.
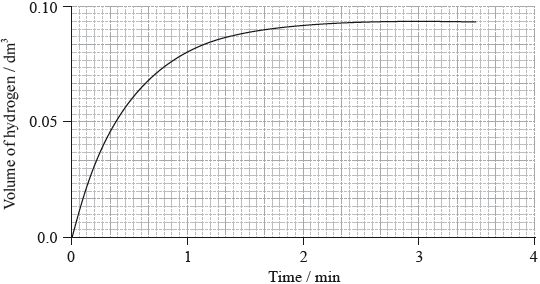
(i) The graph shows the volume of hydrogen produced against time under these experimental conditions.
Sketch two curves, labelled I and II, to show how the volume of hydrogen produced (under the same temperature and pressure) changes with time when:
I. using the same mass of magnesium powder instead of a piece of magnesium ribbon;
II. 0.100 g of magnesium ribbon is added to \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) sulfuric acid.
(ii) Outline why it is better to measure the volume of hydrogen produced against time rather than the loss of mass of reactants against time.
(i) Calculate the amount, in mol, of anhydrous magnesium sulfate.
(ii) Calculate the enthalpy change, \(\Delta {H_1}\), for anhydrous magnesium sulfate dissolving in water, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). State your answer to the correct number of significant figures.
(i) Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\), for the hydration of solid anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}\).
(ii) The literature value for the enthalpy of hydration of anhydrous magnesium sulfate is \( – 103{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). Calculate the percentage difference between the literature value and the value determined from experimental results, giving your answer to one decimal place. (If you did not obtain an answer for the experimental value in (c)(i) then use the value of \( – 100{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\), but this is not the correct value.)
Another group of students experimentally determined an enthalpy of hydration of \( – 95{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). Outline two reasons which may explain the variation between the experimental and literature values.
(i) State the equation for the reaction of sulfuric acid with magnesium carbonate.
(ii) Deduce the Lewis (electron dot) structure of the carbonate ion, giving the shape and the oxygen-carbon-oxygen bond angle.
Lewis (electron dot) structure:
Shape:
Bond angle:
Answer/Explanation
Markscheme
(i) 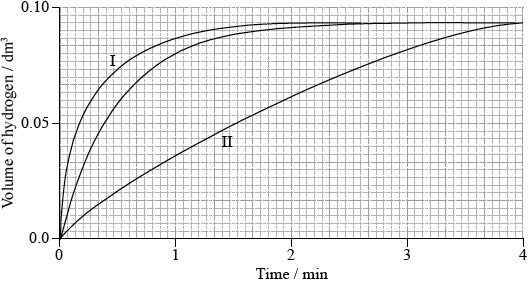
I: line which is steeper/increases faster and finishes at the same height;
II: line which is less steep/increases more slowly and finishes at the same height;
(ii) mass of hydrogen produced is very small (so not accurate) / decrease in mass is very small (so not accurate);
(i) \(n({\text{MgS}}{{\text{O}}_4}) = \left( {\frac{{3.01}}{{120.37}} = } \right){\text{ }}0.0250{\text{ (mol)}}\);
(ii) energy released \( = 50.0 \times 4.18 \times 9.7 \times 2027{\text{ (J)}}/2.027{\text{ (kJ)}}\);
\(\Delta {H_1} = – 81{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
Award [2] for correct answer.
Award [2] if 53.01 is used giving an answer of –86 (kJ mol–1).
Award [1 max] for +81/81/+86/86 (kJ mol−1).
Award [1 max] for –81000/–86000 if units are stated as J mol−1.
Allow answers to 3 significant figures.
(i) \(\Delta H{\text{ }}( = \Delta {H_1} – \Delta {H_2}) = – 99{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}})\);
Award [1] if –86 is used giving an answer of –104 (kJ mol−1).
(ii) \(\frac{{(103 – 99)}}{{103}} \times 100 = 3.9\% \);
Accept answer of 2.9 % if –100 used but only if a value for (b)(i) is not
present.
Award [1] if –104 is used giving an answer of 1.0% .
Accept correct answers which are not to 1 decimal place.
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) not completely anhydrous / OWTTE;
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) is impure;
heat loss to the atmosphere/surroundings;
specific heat capacity of solution is taken as that of pure water;
experiment was done once only so it is not scientific;
density of solution is taken to be \(1{\text{ g}}\,{\text{c}}{{\text{m}}^{ – 3}}\);
mass of \(7{{\text{H}}_2}{\text{O}}\) ignored in calculation;
uncertainty of thermometer is high so temperature change is unreliable;
literature values determined under standard conditions but this experiment is not;
all solid not dissolved;
(i) \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{MgC}}{{\text{O}}_3}{\text{(s)}} \to {\text{MgS}}{{\text{O}}_4}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
Do not accept H2CO3.
(ii) 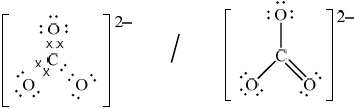 ;
;
Accept crosses, lines or dots as electron pairs.
Accept any correct resonance structure.
Award [0] if structure is drawn without brackets and charge.
Award [0] if lone pairs not shown on O atoms.
shape: trigonal/triangular planar;
bond angle: 120°;
Accept answers trigonal/triangular planar and 120° if M1 incorrect, but no other answer should be given credit.
Examiners report
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Question
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) ethanoic acid were added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
(i) Identify the reagent necessary for this reaction to occur.
(ii) Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
Determine the enthalpy change, in kJ mol\(^{ – 1}\), for this reaction, using Table 10 of the Data Booklet.
Bromoethene, \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHBr}}\), can undergo polymerization. Draw a section of this polymer that contains six carbon atoms.
Answer/Explanation
Markscheme
HCl is a strong acid and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) is a weak acid so HCl has higher conductivity / HCl dissociates completely in water and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) does not, so HCl has higher conductivity / HCl is stronger acid (than \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) so has higher \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and higher conductivity;
(i) \({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{HCO}}_3^ – {\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept NaHCO3(aq) and CH3COONa (aq) instead of ions.
Ignore state symbols.
(ii) \(n{\text{(C}}{{\text{H}}_3}{\text{COOH)}} = 0.00500{\text{ (mol)}}\) and \(n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\({\text{NaHC}}{{\text{O}}_3}\) is limiting;
(iii) \(n{\text{(C}}{{\text{O}}_2}{\text{)}} = n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\(m{\text{(C}}{{\text{O}}_2}{\text{)}} = 0.00450 \times 44.01 = 0.198{\text{ (g)}}\);
Award [2] for correct final answer.
(i) \(T = 363{\text{ K}}\) and \(V = 9.50 \times {10^{ – 5}}{\text{ }}{{\text{m}}^3}\);
Accept V = 9.5 \( \times \) 10–2 dm3 if P is used as 101 kPa in calculation.
\(n = \frac{{PV}}{{RT}} = \frac{{1.01 \times {{10}^5} \times 9.50 \times {{10}^{ – 5}}}}{{8.31 \times 363}}\);
\( = 3.18 \times {10^{ – 3}}{\text{ (mol)}}\);
Award [3] for correct final answer.
(ii) \(M = \left( {\frac{m}{n} = \frac{{0.348}}{{3.18 \times {{10}^{ – 3}}}} = } \right)109{\text{ }}({\text{g}}\,{\text{mo}}{{\text{l}}^{ – 1}})\);
(i) (dilute aqueous) NaOH/sodium hydroxide / KOH/potassium hydroxide;
Do not accept hydroxide/OH–.
(ii) 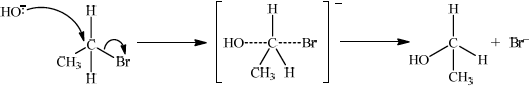
curly arrow going from lone pair/negative charge on O in HO– to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
bonds broken:
1(C=C) \( + 1\) (H–Br) / \((612 + 366 = )978{\text{ (kJ)}}\);
Accept 2630 (kJ).
bonds formed:
1(C–C) \( + 1\) (C–H) \( + 1\) (C–Br) / \((1 \times 347 + 1 \times 413 + 1 \times 290 = )1050{\text{ (kJ)}}\);
Accept 2702 (kJ).
\(\Delta H = – 72{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}})\);
Award [3] for correct final answer.
Award [2 max] for +72 (kJ mol−1).
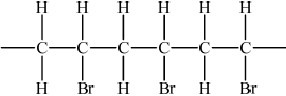 ;
;
Extension bonds required.
Ignore brackets and n.
Examiners report
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ – }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
