Question
An investigation was carried out to determine the effect of chain length of the alcohol on the equilibrium constant, $K_{\mathrm{c}}$, for the reversible reaction:
$$
\mathrm{ROH}+\mathrm{CH}_3 \mathrm{COOH} \stackrel{\mathrm{H}^{+}(\mathrm{aq})}{\rightleftharpoons} \mathrm{CH}_3 \mathrm{COOR}+\mathrm{H}_2 \mathrm{O}
$$
The reactants, products and the catalyst form a homogeneous mixture.
Fixed volumes of each alcohol, the ethanoic acid and the sulfuric acid catalyst were placed in sealed conical flasks.
At equilibrium, the flasks were placed in an ice bath, and samples of each flask titrated with $\mathrm{NaOH}(\mathrm{aq})$ to determine the ethanoic acid concentration present in the equilibrium mixture.
The following processed results were obtained.
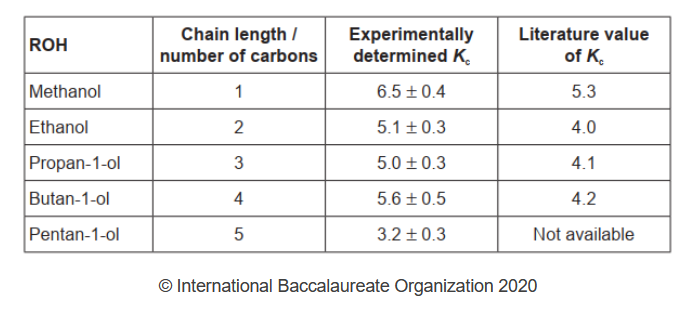
c. Suggest why the titration must be conducted quickly even though a low temperature is maintained.
d. An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with $\mathrm{NaOH}$ (aq). Outline why this experiment was necessary.
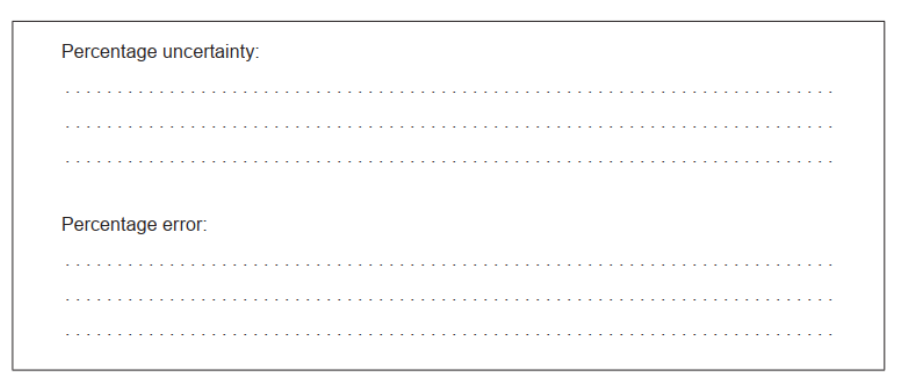
e. Calculate the percentage uncertainty and percentage error in the experimentally determined value of $K_{\mathrm{c}}$ for methanol.
g. Suggest a risk of using sulfuric acid as the catalyst.
▶️Answer/Explanation
Markscheme
a. Independent variable:
chain length $O R$ number of carbon «atoms in alcohol»
AND
Dependent variable:
volume of $\mathrm{NaOH} O R K_c$ /equilibrium constant $O R$ equilibrium concentration/moles of $\mathrm{CH}_3 \mathrm{COOH}$
b. dilution/lower concentrations
less frequent collisions «per unit volume»
Accept “lowers concentration of acid catalyst” for M1. M2 must refer to increase in activation energy or different pathway.
Do not accept responses referring to equilibrium.
c. equilibrium shifts to left
OR
more ethanoic acid is produced «as ethanoic acid is neutralized»
OR
prevents/slows down ester hydrolysis
Accept “prevents equilibrium shift” if described correctly without direction.
d. to determine volume/moles of $\mathrm{NaOH}$ used up by the catalyst/sulfuric acid «in the titration»
OR
to eliminate/reduce «systematic» error caused by acid catalyst
Do not accept “control” OR “standard” alone.
e. Percentage uncertainty:
$$
« \frac{0.4 \times 100}{6.5}=» 6 \ll \% »
$$
Percentage error:
$$
« \frac{6.5-5.3}{5.3}=» 23 \ll \% »
$$
Award [1 max] if calculations are reversed OR if incorrect alcohol is used.
f. Any two:
large percentage error means large systematic error «in procedure» small percentage uncertainty means small random errors random errors smaller than systematic error
Award [2] for “both random and systematic errors are significant.”
g. corrosive/burns/irritant/strong oxidizing agent/carcinogenic
OR
disposal is an environmental issue
OR
causes other side reactions/dehydration/decomposition
Do not accept just “risk of accidents” OR “health risks” OR “hazardous”.
Question
Body fluids have different $\mathrm{pH}$ values.
a. Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
b. An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
c. Outline how ranitidine reduces stomach acidity.
d. Calculate the $\mathrm{pH}$ of a buffer solution which contains $0.20 \mathrm{~mol} \mathrm{dm}^{-3}$ ethanoic acid and $0.50 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium ethanoate. Use section 1 of the data booklet.
$$
\mathrm{p} K_{\mathrm{a}}(\text { ethanoic acid })=4.76
$$
▶️Answer/Explanation
Markscheme
a. hydrochloric acid/ $\mathrm{HCl}$ «(aq)» $A N D$ strong «acid»
b. $\mathrm{MgCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}($
(l)
NOTE: Accept ionic equation.
c. blocks/binds to H2-histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to H2-histamine receptors «and triggering acid secretion»
OR
prevents parietal cells from releasing/producing acid
NOTE: Do not accept “antihistamine” by itself.
Accept “H2-receptor antagonist/H2RA” OR “blocks/inhibits action of histamine”.
Accept “blocks receptors in parietal cells “from releasing/producing acid»”.
Do not accept “proton pump/ATPase inhibitor”.
d. $« p K_{\mathrm{a}}=4.76 »$
$$
\begin{aligned}
& « \mathrm{pH}=\mathrm{p} K_{\mathrm{a}}+\log \left(\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\right) » \\
& « \mathrm{pH}=4.76+0.40=\text { 5.16 }
\end{aligned}
$$
Question
A student wished to determine the concentration of a solution of sodium hydroxide by titrating it against a 0.100moldm−3 aqueous solution of hydrochloric acid.
4.00g of sodium hydroxide pellets were used to make 1.00dm3 aqueous solution.
20.0cm3 samples of the sodium hydroxide solution were titrated using bromothymol blue as the indicator.
Outline, giving your reasons, how you would carefully prepare the 1.00dm3 aqueous solution from the 4.00g sodium hydroxide pellets.
(i) State the colour change of the indicator that the student would see during his titration using section 22 of the data booklet.
(ii) The student added the acid too quickly. Outline, giving your reason, how this could have affected the calculated concentration.
Suggest why, despite preparing the solution and performing the titrations very carefully, widely different results were obtained.
▶️Answer/Explanation
Markscheme
Key Procedural Steps:
use volumetric flask
mix the solution
fill up to line/mark/«bottom of» meniscus/1 dm3 «with deionized/distilled water»
Key Technique Aspects:
use balance that reads to two decimal places/use analytical balance/use balance of high precision
mix pellets in beaker with deionized/distilled water «and stir with glass rod to dissolve»
use a funnel «and glass-rod» to avoid loss of solution
need to rinse «the beaker, funnel and glass rod» and transfer washings to the «volumetric» flask
Safety Precautions:
NaOH corrosive/reacts with water exothermically
keep NaOH in dessicator
let the solution cool
Two marks may be awarded from two different categories or from within one category.
Do not accept “use of a funnel to transfer the solid”.
Do not accept “keep volumetric flask in cold water/ice”.
(i) blue to green/yellow
(ii) equivalence point has been exceeded
OR
greater volume of/too much acid has been added
«calculated» concentration increased
Accept “end-point” for “equivalence point”.
colour difficult to detect
OR
using different HCl standards
OR
no significant figures used in subsequent calculation
OR
incorrect method of calculation
Accept any valid hypothesis.
Do not accept any mistakes associated with techniques (based on stem of question) eg. parallax error, not rinsing glassware, etc.
Do not accept “HCl was not standardized”.
Accept “reaction of NaOH with CO2 «from air»”.
Accept “NaOH hygroscopic/absorbs moisture/H2O «from the air/atmosphere»”.
Accept “impurities in NaOH”.
Accept “temperature changes during experiment”.
Ignore a general reference to random errors.
Question
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
Deduce why more heat was produced in mixture B than in mixture A.
Deduce why the temperature is higher in mixture C than in mixture D.
▶️Answer/Explanation
Markscheme
more «moles/amount of» acid have been added/reacted
OR
more of the limiting reagent is present
OR
more «of the exothermic» reaction has occurred
[1 mark]
no more reaction/same energy released AND cold/colder/cooler liquid added
OR
no more reaction/same energy released AND greater total volume of liquid
Accept “no more reaction/same energy released AND greater heat loss «to the surroundings in mixture D»”.
[1 mark]
Question
Antacids react with hydrochloric acid in the stomach to relieve indigestion. A student investigated different brands of antacid to see which caused the largest increase in pH in a given time. She added the antacids to hydrochloric acid, and recorded the change in pH over five minutes.
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
Suggest two variables, besides the time of reaction, which the student should have controlled in the experiment to ensure a fair comparison of the antacids.
Calculate the uncertainty in the change in pH.
The student concluded that antacid B was the most effective, followed by A then C and finally D. Discuss two arguments that reduce the validity of the conclusion.
▶️Answer/Explanation
Markscheme
Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O (l)
Accept full or net ionic equation.
Any two from:
volume «of HCl»
concentration «of HCl»/[HCl]
temperature «of HCl»
mass of antacid/tablets
size of antacid particles/tablets
OR
surface area of antacid «particles»/tablets
Accept “number of tablets/different doses”.
Do not accept “same pH meter” OR “initial pH” OR “concentration of antacid/[antacid]”.
A variable must be given so do not accept answers such as “stirring”, “whether tablets are whole or crushed” etc.
[Max 2 Marks]
(±) 0.04
OR
(±) 0.03
Any two of:
uncertainty «(±)0.04/(±)0.03» means A and C cannot be distinguished
each measurement was conducted once
stomach pH should not be raised a lot «so antacid B is not necessarily effective»
mass/number of tablets/dose «of antacid» used was not controlled
actual environment in stomach is different
Accept “amount of tablets” for “dose”.
Do not accept “nature/composition of tablets differs”.
Accept an answer such as “time frame is too short since some antacids could be long-acting drugs if they contain a gelatinisation/delaying agent” but not just “time frame is too short since some antacids could be long-acting drugs”.
[Max 2 Marks]

