Question
\text { EDTA is produced by reacting ethane-1,2-diamine with chloroethanoic acid, } \mathrm{ClCH}_2 \mathrm{COOH} \text {. }
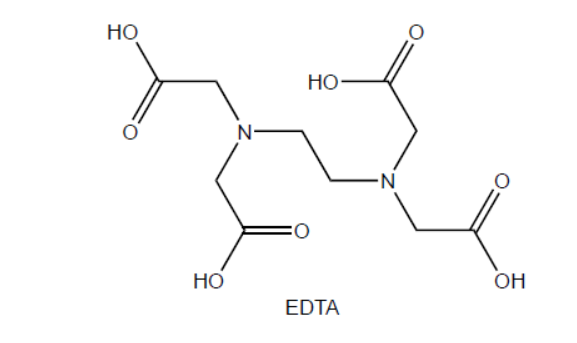
a. Identify the other product formed.
b. Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
▶️Answer/Explanation
Markscheme
a. $\mathrm{HCl} /$ hydrogen chloride
Accept “hydrochloric acid”.
b. forms four/six/several/multiple coordinate/coordination bonds «to a central metal ion»
OR
is a polydentate/tetradentate/hexadentate ligand
forms more stable complex/stronger bonds with central metal ion OR
increases entropy/S by releasing smaller «monodentate ligand» molecules previously complexed
complex ions are much larger «and can be removed easily due to large size of chelate complexes» OR
heavy metal ions trapped inside the ligand/become «biologically» inactive/nontoxic/harmless
Accept “dative “covalent»” for “coordinate/coordination”.
Do not accept just “chelates”.
Question
Body fluids have different $\mathrm{pH}$ values.
a. Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
b. An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
c. Outline how ranitidine reduces stomach acidity.
d. Calculate the $\mathrm{pH}$ of a buffer solution which contains $0.20 \mathrm{~mol} \mathrm{dm}^{-3}$ ethanoic acid and $0.50 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium ethanoate. Use section 1 of the data booklet.
$\mathrm{p} K_{\mathrm{a}}($ ethanoic acid $)=4.76$
▶️Answer/Explanation
Markscheme
a. hydrochloric acid/ $\mathrm{HCl}$ «(aq)» $A N D$ strong «acid»
b. $\mathrm{MgCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})$
NOTE: Accept ionic equation.
c. blocks/binds to H2-histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to H2-histamine receptors «and triggering acid secretion»
OR
prevents parietal cells from releasing/producing acid
NOTE: Do not accept “antihistamine” by itself.
Accept “H2-receptor antagonist/H2RA” OR “blocks/inhibits action of histamine”.
Accept “blocks receptors in parietal cells “from releasing/producing acid””.
Do not accept “proton pump/ATPase inhibitor”.
d. $« p K_{\mathrm{a}}=4.76 »$
$$
\begin{aligned}
& \text { «pH }=\mathrm{p} K_{\mathrm{a}}+\log \left(\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\right) » \\
& « \mathrm{pH}=4.76+0.40=» 5.16
\end{aligned}
$$
Question
The cumene process is used for the production of both propanone and phenol. The overall reaction is shown in the equation below.
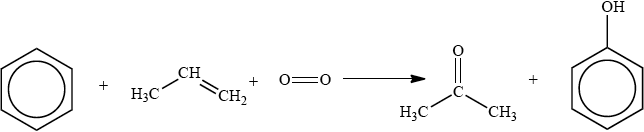
This process is important in the polymer industry. Propanone can be converted into methyl methacrylate, the monomer used to make Perspex®, and phenol is used in phenol-methanal resins, which are important thermosetting plastics.
State and explain how the presence of a halogen substituent might affect the acidity of carboxylic acids.
Propanone could also be formed from propene by reaction with steam over an acidic catalyst, followed by oxidation of the product.
The reaction of propene with water can yield two possible products. Explain, in terms of the stability of the intermediate carbocations, why one is formed in much greater quantities than the other.
▶️Answer/Explanation
Markscheme
halogens make them more acidic;
halogens are electron withdrawing;
Accept halogens (can be) electronegative.
reduces charge on/stabilizes anion formed / weakens O–H bond / makes it easier to lose \({{\text{H}}^ + }\) ion;
Accept decreases pKa.
Accept causes anion to be weaker base.
one product involves a primary carbocation and other a secondary carbocation;
secondary carbocation is more stable (than the primary carbocation, and hence this produces the major product);
alkyl groups reduce charge on carbon atom (through an inductive effect);
Positive inductive effect of alkyl groups alone not enough for M3.
Examiners report
(a) (i) was well done by the better candidates only, but most candidates only scored one mark in (ii) and no marks in (iii).
(d) was very poorly answered. Some knew that there was an inductive effect but did not understand what this meant, namely that through the positive inductive effect the alkyl groups reduce the charge on the carbon atom.
Question
The combustion of fossil fuels produces large amounts of CO2, a greenhouse gas.
The diagram below illustrates a range of wavelengths in the electromagnetic spectrum.
Synthesis gas, or syngas, mainly composed of CO(g) and H2(g), is an alternative form of fuel. It can be produced by coal or biomass gasification, passing steam over the source material in a low oxygen environment.
Identify which region, A or B, corresponds to each type of radiation by completing the table.
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) \( \rightleftharpoons \) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
Suggest an equation for the production of syngas from coal.
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
Suggest a reason why syngas may be considered a viable alternative to crude oil.
▶️Answer/Explanation
Markscheme
Accept “B” alone for incoming radiation from sun.
All three correct answers necessary for mark.
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)
State symbols AND equilibrium arrow required for mark.
Accept
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq).
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq).
[1 mark]
CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
OR
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + H2O(l) \( \rightleftharpoons \) H3O+(aq) + HCO3–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
H2CO3(aq) + 2H2O(l) \( \rightleftharpoons \) 2H3O+(aq) + CO32–(aq)
OR
H2CO3(aq) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq)
equilibrium shifts to the right causing increase in [H3O+]/[H+ ] «thereby decreasing pH»
Equilibrium sign needed in (b) (ii) but penalize missing equilibrium sign once only in b (i) and (ii).
Do not accept “CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq)” unless equation was not given in b (i).
[2 marks]
C(s) + H2O(g) → CO(g) + H2(g)
OR
3C(s) + H2O(g) + O2(g) → 3CO(g) + H2(g)
OR
4C(s) + 2H2O(g) + O2(g) → 4CO(g) + 2H2(g)
OR
5C(s) + H2O(g) + 2O2(g) → 5CO(g) + H2(g)
Accept other correctly balanced equations which produce both CO AND H2.
[1 mark]
8CO(g) + 17H2(g) → C8H18(l) + 8H2O(g)
[1 mark]
coal more plentiful than crude oil
OR
syngas can be produced from biomass/renewable source
OR
syngas can undergo liquefaction to form octanes/no need to transport crude
OR
syngas can be produced by gasification underground, using carbon
OR
capture/storage «to not release CO2 to the atmosphere»
OR
coal gasification produces other usable products/slag
[1 mark]

