Question
Calcium nitrate contains both covalent and ionic bonds.
Nitrogen also forms oxides, which are atmospheric pollutants.
State the formula of both ions present and the nature of the force between these ions.
Ions:
Nature of force:[2]
State which atoms are covalently bonded.[1]
Bonding in the nitrate ion involves electron delocalization. Explain the meaning of electron delocalization and how it affects the ion.[2]
Outline the source of these oxides.[1]
State one product formed from their reaction with water.[1]
State one environmental problem caused by these atmospheric pollutants.[1]
Answer/Explanation
Markscheme
\({\text{C}}{{\text{a}}^{2 + }}\) and \({\text{NO}}_3^ – \);
electrostatic (attraction);
Do not accept ionic.
nitrogen/N and oxygen/O;
Do not accept nitrate/NO3–.
Accept atoms in nitrate/NO3–
pi/\(\underline \pi \)-electrons shared by more than two atoms/nuclei / a pi/\(\underline \pi \)-bond/overlapping p-orbitals that extends over more than two atoms/nuclei;
all (N–O) bonds equal length/strength/bond-order / charge on all oxygen/O atoms equal / increases stability/lowers PE (of the ion);
Accept a diagram that clearly shows one or both points.
produced by high temperature combustion;
Accept combustion/jet/car engines / car exhaust/emissions / lightning / action of bacteria/microorganisms.
Do not accept combustion/burning, cars, planes, jets, factories, power plants etc.
nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\) / nitrous acid/nitric(III) acid/\({\text{HN}}{{\text{O}}_{\text{2}}}\);
Accept “form acidic solutions / acid rain”.
acid deposition/rain / respiratory problems / corrosion problems / decomposition of ozone layer / photochemical smog / acidification/pollution of lakes / damage to plants/ trees;
Accept “acid rain” in either part (ii) or part (iii) but not both.
Do not accept air pollution.
Examiners report
It was distressing how many students taking HL Chemistry (over 50%?) do not know the formula of the nitrate ion! Many students also gave the type of bonding present between the ions, rather than the nature of the force asked for, though almost all could correctly identify the covalently bonded atoms. Hardly any could explain delocalization in terms of the overlap of p-orbitals, or the extension of a \(\pi \)-bond, over more than two atoms, though its effect on structure and stability were better known. In part (c), which tested Aim 8 of the syllabus, most managed to gain some of the marks available for atmospheric pollution from oxides of nitrogen. Inevitably, owing to some overlap in assessment statements these concepts would be more familiar to those studying the Environmental Chemistry option, but undoubtedly studying other options assists in other areas, such as organic chemistry.
Question
Graphing is an important tool in the study of rates of chemical reactions.
The graph represents the titration of 25.00 cm3 of 0.100 mol dm−3 aqueous ethanoic acid with 0.100 mol dm−3 aqueous sodium hydroxide.
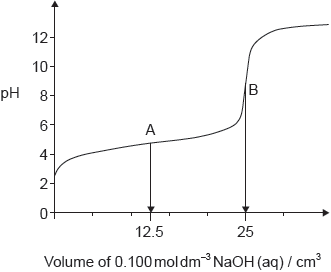
Deduce the major species, other than water and sodium ions, present at points A and B during the titration.
[2]
Calculate the pH of 0.100 mol dm−3 aqueous ethanoic acid.
Ka = 1.74 × 10−5[2]
Outline, using an equation, why sodium ethanoate is basic.[1]
Predict whether the pH of an aqueous solution of ammonium chloride will be greater than, equal to or less than 7 at 298 K.[1]
Formulate the equation for the reaction of nitrogen dioxide, NO2, with water to form two acids.[1]
Formulate the equation for the reaction of one of the acids produced in (e)(i) with calcium carbonate.[1]
Answer/Explanation
Markscheme
A: CH3COOH/ethanoic/acetic acid AND CH3COO–/ethanoate/acetate ions
B: CH3COO–/ethanoate/acetate ions
Penalize “sodium ethanoate/acetate” instead of “ethanoate/acetate ions” only once.
[2 marks]
\({K_{\text{a}}} = 1.74 \times {10^{ – 5}} = \frac{{{{{\text{[}}{{\text{H}}^ + }{\text{]}}}^2}}}{{0.10}}\)
OR
[H+] = 1.32 × 10–3 «mol dm–3»
«pH =» 2.88
Accept [2] for correct final answer.
[2 marks]
«forms weak acid and strong base, thus basic»
CH3COO–(aq) + H2O(l) \( \rightleftharpoons \) CH3COOH(aq) + OH–(aq)
Accept → for \( \rightleftharpoons \).
[1 mark]
less than 7
[1 mark]
2NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq)
[1 mark]
2HNO2(aq) + CaCO3(s) → Ca(NO2)2(aq) + CO2(g) + H2O(l)
OR
2HNO3(aq) + CaCO3(s) → Ca(NO3)2(aq) + CO2(g) + H2O(l)
[1 mark]
