Question
(i) State the oxidation state of manganese in MnO2 and MnCl2. [2]
MnO2:
MnCl2:
(ii) Deduce, referring to oxidation states, whether MnO2 is an oxidizing or reducing agent. [1]
Answer/Explanation
Ans
i
MnO2: +4
MnCl2: +2
ii oxidizing agent AND oxidation state of Mn changes from +4 to +2/decreases
Question
The percentage of iron(II) ions, \({\text{F}}{{\text{e}}^{2 + }}\), in a vitamin tablet can be estimated by dissolving the tablet in dilute sulfuric acid and titrating with standard potassium manganate(VII) solution, \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). During the process iron(II) is oxidized to iron(III) and the manganate(VII) ion is reduced to the manganese(II) ion, \({\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}}\). It was found that one tablet with a mass of 1.43 g required \({\text{11.6 c}}{{\text{m}}^{\text{3}}}\) of \(2.00 \times {10^{ – 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) to reach the end-point.
State the half-equation for the oxidation of the iron(II) ions.[1]
State the half-equation for the reduction of the \({\text{MnO}}_4^ – \) ions in acidic solution.[1]
Deduce the overall redox equation for the reaction.[1]
Calculate the amount, in moles, of \({\text{MnO}}_4^ – \) ions present in \({\text{11.6 c}}{{\text{m}}^{\text{3}}}\) of \(2.00 \times {10^{ – 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\).[1]
Calculate the amount, in moles, of \({\text{F}}{{\text{e}}^{2 + }}\) ions present in the vitamin tablet.[1]
Determine the percentage by mass of \({\text{F}}{{\text{e}}^{2 + }}\) ions present in the vitamin tablet.[2]
Answer/Explanation
Markscheme
\({\text{F}}{{\text{e}}^{2 + }} \to {\text{F}}{{\text{e}}^{3 + }} + {{\text{e}}^ – }\);
\({\text{MnO}}_4^ – + {\text{8}}{{\text{H}}^ + } + {\text{5}}{{\text{e}}^ – } \to {\text{M}}{{\text{n}}^{2 + }} + {\text{4}}{{\text{H}}_2}{\text{O}}\);
\({\text{MnO}}_4^ – + {\text{5F}}{{\text{e}}^{2 + }} + {\text{8}}{{\text{H}}^ + } \to {\text{M}}{{\text{n}}^{2 + }} + {\text{5F}}{{\text{e}}^{3 + }} + {\text{4}}{{\text{H}}_2}{\text{O}}\);
Accept e instead of e–.
\({\text{amount of MnO}}_4^ – = \frac{{11.6}}{{1000}} \times 0.0200 = 2.32 \times {10^{ – 4}}{\text{ mol}}\);
\({\text{amount of F}}{{\text{e}}^{2 + }} = 5 \times 2.32 \times {10^{ – 4}} = 1.16 \times {10^{ – 3}}{\text{ mol}}\);
\({\text{mass of F}}{{\text{e}}^{2 + }} = 55.85 \times 1.16 \times {10^{ – 3}} = 6.48 \times {10^{ – 2}}{\text{ g}}\);
\({\text{percentage of F}}{{\text{e}}^{2 + }}{\text{ in tablet}} = \frac{{6.48 \times {{10}^{ – 2}}}}{{1.43}} = 100 = 4.53\% \);
Examiners report
This question was generally well answered. A common mistake with writing half-equations was the failure to realise that only single arrows should be used if oxidation and reduction are specifically asked for. Candidates were only penalized once for this error.
Given that the half-equation involving \({\text{MnO}}_4^ – \) ions is provided in the Data Booklet, it was surprising that several candidates could not correctly write the equation for their reduction in acidic solution.
Question
Chromium is a typical transition metal with many uses.
A voltaic cell is constructed as follows. One half-cell contains a platinum electrode in a solution containing \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\) and \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\). The other half-cell contains an iron electrode in a solution containing \({\text{F}}{{\text{e}}^{2 + }}\) ions. The two electrodes are connected to a voltmeter and the two solutions by a salt bridge.
Distinguish between the terms oxidation and reduction in terms of oxidation numbers.[1]
State the names of \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) and \({\text{Cr}}{{\text{O}}_{\text{3}}}\).
\({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
\({\text{Cr}}{{\text{O}}_{\text{3}}}\):[2]
Define the term oxidizing agent.[1]
\({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 – }{\text{(aq)}}\) and \({{\text{I}}^ – }{\text{(aq)}}\) ions react together in the presence of acid to form \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) and \({\text{IO}}_3^ – {\text{(aq)}}\) ions. Deduce the balanced chemical equation for this redox reaction and identify the species that acts as the oxidizing agent.[3]
Draw a diagram of the voltaic cell, labelling the positive and negative electrodes (cathode and anode) and showing the direction of movement of the electrons and ions. Deduce an equation for the reaction occurring in each of the half-cells, and the equation for the overall cell reaction.[5]
Define the term standard electrode potential.[1]
Calculate the cell potential, in V, under standard conditions, using information from Table 14 of the Data Booklet.[1]
State two characteristic properties of transition elements.[2]
State the type of bond formed by a ligand and identify the feature that enables it to form this bond.[2]
Explain why the complex \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{3 + }}}}\) is coloured.[3]
Draw an orbital box diagram (arrow-in-box notation) showing the electrons in the 4s and 3d sub-levels in chromium metal.[1]
Chromium is often used in electroplating. State what is used as the positive electrode (anode), the negative electrode (cathode) and the electrolyte in the chromium electroplating process.[3]
Answer/Explanation
Markscheme
Oxidation: increase in oxidation number and Reduction: decrease in oxidation number / OWTTE;
Cr2O3:
chromium(III) oxide;
Do not award mark for chromium oxide.
CrO3:
chromium(VI) oxide;
Do not award mark for chromium oxide.
Do not award any marks if chromium oxide without Roman numerals is given for both.
substance reduced / causes other substance to be oxidized / increase oxidation number of another species / gains electrons / OWTTE;
Oxidizing agent:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }\) / dichromate (ion);
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {{\text{I}}^ – }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ – {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\)
Award [1] for coefficients: Cr2O72–(aq), I–(aq), 2Cr3+(aq), IO3–(aq).
Award [1] for coefficients: 8H+(aq), 4H2O(l).
Award [1 max] if coefficients of reactants only correct i.e. Cr2O72–, I– and 8H+.
Award [1 max] if coefficients of products only correct i.e. 2Cr3+, IO3– and 4H2O.
Award [1 max] for correct reactants and products.
Ignore state symbols.
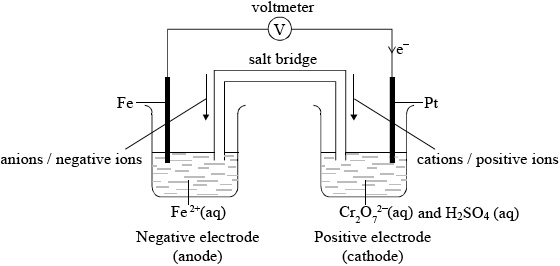
Voltaic cell showing:
labelled positive electrode (cathode) and negative electrode (anode);
direction of electrons in external circuit and direction of ions in salt bridge;
Award mark if correct direction of electrons is indicated but e– not labelled in external circuit.
Allow e instead of e–.
Cations/positive ions and anions/negative ions must be identified in salt bridge. Allow correct movement of ions in electrolyte instead of movement of ions in salt bridge (e.g. Fe2+ from Fe at negative electrode/anode etc.).
If both movement of ions in salt bridge and movement of ions in electrolyte is given but one is incorrect do not award mark.
Positive electrode (cathode):
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ – } \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\);
Negative electrode (anode):
\({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – }\);
Penalize once only.
Penalize once only if electrodes or equations reversed.
For both electrodes allow e instead of e–.
Overall cell reaction:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {\text{3Fe(s)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{3F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols throughout (d) (i).
potential under standard conditions relative to standard hydrogen electrode/SHE;
Reference must be made to standard conditions.
Instead of standard conditions allow either solute concentration of 1 mol\(\,\)dm–3/1 M/1 mol\(\,\)L–1 or 100 kPa/105 Pa for gases.
Allow 1 bar for 100 kPa/105 Pa.
Allow 1 atm/1.01 \( \times \) 105 Pa.
Allow voltage instead of potential.
\(( + )1.78{\text{ (V)}}\);
catalysts;
variable oxidation state/numbers;
Allow variable valency.
magnetic (properties);
(form) coloured ions/compounds;
Allow just coloured.
(form) complexes/complex ions;
Allow other metallic physical properties such as high densities/high melting points etc.
Allow partially filled/incomplete d subshell/sub-level.
dative (covalent)/coordinate;
Lewis base / (species/ion/molecule with) lone/non-bonding pair;
partially filled/incomplete d subshell/sub-level/orbitals;
d orbitals split (into two sets of different energies);
colour due to electron transition between (split) d orbitals / frequencies of visible light absorbed by electrons moving from lower to higher d levels;
colour due to remaining frequencies / complementary colour seen;
Allow wavelength as well as frequency.
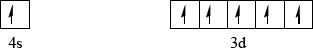
Accept half-arrows or full arrows and boxes in reverse order.
Do not penalize if additional sub-levels are shown, if sub-levels are not labelled or if no boxes are drawn (providing system of arrows correct).
Do not award mark if sub-levels are incorrectly labelled.
Orbital diagram may also be represented with sub-levels shown at different relative energy positions.
Positive electrode (anode): chromium;
Allow lead/titanium/platinum/graphite.
Negative electrode (cathode): object to be plated;
Allow specific example here e.g. spoon.
Electrolyte: \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\);
Allow (mixture of) \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) and CrO42–(aq)/chromate/chromic acid/H2CrO4.
Ignore state symbols.
Allow any soluble salt of Cr3+.
Examiners report
Candidates generally knew that oxidation involves an increase in oxidation number and reduction a decrease.
Some forgot to include the Roman Numerals here and a large majority simply got the Roman Numeral incorrect. One G2 comment suggested that it would have been better if systematic was included in the question which is a fair point, though typically candidates simply put chromium oxide for both compounds which showed misunderstanding of what was really required.
The definition of an oxidizing agent was well answered.
Most candidates knew that the dichromate ion acted as the oxidizing agent but many made lots of errors in deducing the balanced chemical equation.
Only the best candidates scored all five marks, though most candidates scored at least two marks. Some candidates mixed up the cathode and anode. Equilibrium signs were often written and very few gave the correct direction of the movement of ions. Some G2 comments stated that is was not clear what ion movement was required – flow of ions through the salt bridge or just movement of ions towards the electrodes in the electrolyte. In fact most candidates could not write either and the markscheme in fact allowed credit for either of these to be fair to candidates.
Standard conditions often was omitted.
(iii) was well answered.
Most candidates scored full marks here.
Most candidates scored full marks here.
Many candidates scored two out of three marks.
Many candidates put two electrons in the 4s level and four electrons in the 3d level which was incorrect in the orbital diagram.
Candidates often scored two out of three marks here with the most common error relating to the electrolyte.
