Question
Ethanol was electrolysed at different voltages. The products at the anode, ethanoic acid, ethanal and carbon dioxide, were collected and analysed.
The percentages of products obtained using three different catalysts mounted on a carbon anode, platinum (Pt/C), platinum and ruthenium alloy $(\mathrm{PtRu} / \mathrm{C})$ and platinum and tin alloy $(\mathrm{PtSn} / \mathrm{C})$ are shown.
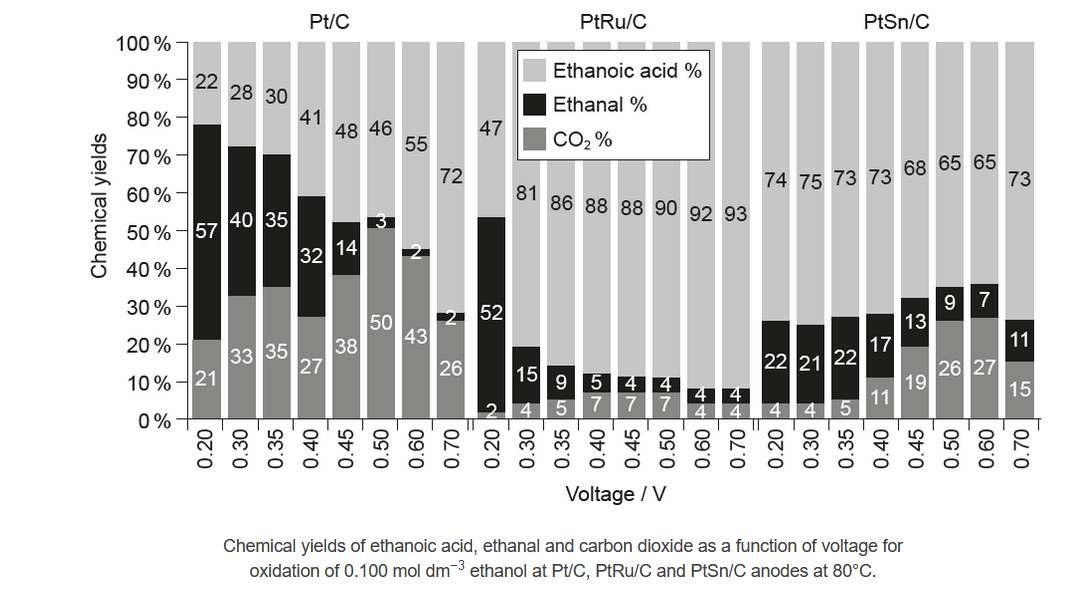
a(i)Describe the effect of increasing the voltage on the chemical yield of:
Ethanal using Pt/C:
Carbon dioxide using PtRu/C:
a(iiDetermine the change in the average oxidation state of carbon.
From ethanol to ethanal:
From ethanol to carbon dioxide:
a(iii)ist the three products at the anode from the least to the most oxidized.
b. Deduce, giving your reason, which catalyst is most effective at fully oxidizing ethanol.
▶️Answer/Explanation
Markscheme
a(i)Ethanal using Pt/C:
decreases
Carbon dioxide using PtRu/C:
“generally» increases $A N D$ then decreases
NOTE: Accept “no clear trend/pattern” OR “increases and decreases” OR “increases, reaches a plateau and “then” decreases” for M2.
a(ii)From ethanol to ethanal:
-2 to -1
OR
$+1 /$ increases by 1
NOTE: Do not accept “2- to 1-“.
From ethanol to carbon dioxide:
-2 to +4
OR
$+6 /$ increase by 6
NOTE: Do not accept “2- to 4+”.
Do not penalize incorrect notation twice.
Penalize incorrect oxidation state value of carbon in ethanol once only.
a(iiięthanal < ethanoic acid < carbon dioxide
NOTE: Accept formulas.
No ECF from 2aii calculations.
b. Pt/platinum/PtC AND highest yield of $\mathrm{CO}_2$ «at all voltages»
NOTE: ECF from 2aiii.
Question
Metals are extracted from their ores by various means.
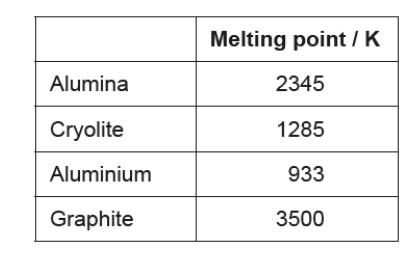
Aluminium is produced by the electrolysis of alumina (aluminium oxide) dissolved in cryolite.
a. Discuss why different methods of reduction are needed to extract metals.
$b(i)$ Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
b(ii)Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
▶️Answer/Explanation
Markscheme
a. ions of more reactive metals are harder to reduce
OR
more reactive metals have more negative electrode potentials
electrolysis is needed/used for most reactive metals
OR
carbon is used to reduce metal oxides of intermediate reactivity/less reactive than carbon
$O R$
heating ore is sufficient for less reactive metals
NOTE: Award [1 max] for ” “uease of reduction/extraction” depends on reactivity”.
$\mathrm{b}$ (i)electronegativity difference $=1.8$ «and average electronegativity $=2.5$ »
$57 \ll \% »$
NOTE: Accept any value in the range 52-65\%.
Award [2] for correct final answer.
b(ii)Anode (positive electrode):
$$
2 \mathrm{O}^{2-} \rightarrow 4 \mathrm{e}^{-}+\mathrm{O}_2(\mathrm{~g})
$$
OR
$$
2 \mathrm{O}^{2-}+\mathrm{C} \rightarrow 4 \mathrm{e}^{-}+\mathrm{CO}_2(\mathrm{~g})
$$
NOTE: Award [1 max] for M1 and M2 if correct half-equations are given at the wrong electrodes OR if incorrect reversed half-equations are given at the correct electrodes.
Cathode (negative electrode):
$$
\mathrm{Al}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}(\mathrm{I})
$$
$\mathrm{O}_2$ gas $\boldsymbol{A N D}$ Al liquid $\boldsymbol{V}$
NOTE: Only state symbols of products required, which might be written as (g) and (I) in half-equations. Ignore any incorrect or missing state symbols for reactants.
Question
Ethanol is a depressant that is widely consumed in many societies. When consumed excessively it has a major impact on families and society as a whole. Other depressants such as diazepam (Valium®) may be prescribed by a doctor.
One problem associated with ethanol consumption is an increased risk of traffic accidents. Police in many countries use a breathalyser to test drivers. The breathalyser contains potassium dichromate(VI).
Describe the colour change of potassium dichromate(VI) when it reacts with ethanol.
State with a reason whether chromium in potassium dichromate(VI) is oxidised or reduced by ethanol.
▶️Answer/Explanation
Markscheme
orange to green;
reduced because oxidation number of Cr decreases / Cr gains electrons;
Explanation needed for mark.
Examiners report
[N/A]
Candidates frequently confused oxidation and reduction or failed to provide a reason as to whether the chromium was oxidised or reduced by ethanol. This highlighted, again, the need for candidates to answer all parts of the question.
Question
Water purity is often assessed by reference to its oxygen content.
The Winkler method uses redox reactions to find the concentration of oxygen in water. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of water was taken from a river and analysed using this method. The reactions taking place are summarized below.
\[\begin{array}{*{20}{l}} {{\text{Step 1}}}&{{\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ – }{\text{(aq)}} + {{\text{O}}_2}{\text{(aq)}} \to {\text{2Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 2}}}&{{\text{Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 3}}}&{{\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 – }{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} \to {{\text{S}}_4}{\text{O}}_6^{2 – }{\text{(aq)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}}} \end{array}\]
Outline the meaning of the term biochemical oxygen demand (BOD).
State what happened to the \({{\text{O}}_{\text{2}}}\) in step 1 in terms of electrons.
State the change in oxidation number for manganese in step 2.
0.0002 moles of \({{\text{I}}^ – }\) were formed in step 3. Calculate the amount, in moles, of oxygen, \({{\text{O}}_{\text{2}}}\), dissolved in water.
▶️Answer/Explanation
Markscheme
amount of oxygen needed to decompose organic matter;
in a specified time/five days / at a specified temp/ 20 °C;
Second mark can only be awarded if reasonable attempt made to define BOD.
gained electrons;
+4 to +2 / decrease by 2;
\(0.00005/5 \times {10^{ – 5}}\) (moles);
Examiners report
In part (a) the term biochemical oxygen demand (BOD) was not well known. Very few candidates could explain that it is related to the level of organic waste in the water measured at a specific temperature for a specific time period.
Many candidates understood that oxygen gained electrons.
Many candidates understood that the oxidation number of manganese dropped from +4 to +2.
Many candidates understood that oxygen gained electrons in (c) (i) and that the oxidation number of manganese dropped from +4 to +2 in (ii). However, they struggled to calculate the moles of dissolved oxygen.
Question
Rechargeable nickel-cadmium batteries are used in portable electrical equipment and emergency lighting.
The discharge process can be summarized by the equation below.
\[{\text{2NiO(OH)(s)}} + {\text{Cd(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{2Ni(OH}}{{\text{)}}_{\text{2}}}{\text{(s)}} + {\text{Cd(OH}}{{\text{)}}_{\text{2}}}{\text{(s)}}\]
State the change in oxidation number of the cadmium and deduce if it is acting as the positive or negative electrode during the discharge process.
Identify a physical property of Cd(OH)2 which allows this process to be reversed and the battery recharged.
▶️Answer/Explanation
Markscheme
\(0 \to + 2\) / increase by 2;
negative;
If decrease by 2, positive, award [1]. If decrease by 2, negative, award [0].
insoluble (\({\text{C}}{{\text{d}}^{2 + }}\) ions do not escape into solution);
Do not accept solid.
Examiners report
Most candidates were able to identify the change of oxidation number of cadmium but very few identified the insolubility of cadmium hydroxide as the physical property which allows the process to be reversed.
Most candidates were able to identify the change of oxidation number of cadmium but very few identified the insolubility of cadmium hydroxide as the physical property which allows the process to be reversed.
Question
The biochemical oxygen demand (BOD) is a measure of water pollution.
State what is meant by the term biochemical oxygen demand (BOD).
▶️Answer/Explanation
Markscheme
amount of oxygen needed to decompose organic matter (in water sample);
in a specified time/five days / at a specified temperature/ 20 °C;
Examiners report
Most candidates knew the meaning of BOD.
Question
Depressants can have different effects depending on their doses.
A breathalyser containing crystals of potassium dichromate(VI) can be used by the police to detect whether a driver has consumed alcohol.
State the chemical formula for potassium dichromate(VI).
Describe the colour change observed during its reaction with ethanol.
State the oxidation number of chromium in the product.
Deduce the full balanced chemical equation for the redox reaction of ethanol with acidified potassium dichromate(VI).
State the name of the organic product formed.
An intoximeter is used to determine an accurate value for the concentration of ethanol in the breath. Explain one technique used for the detection of ethanol in an intoximeter.
▶️Answer/Explanation
Markscheme
\({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\);
orange to green;
Allow yellow instead of orange.
+3/III;
Do not allow incorrect notation such as 3+ or 3.
\({\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{2C}}{{\text{r}}_2}{\text{O}}_7^{2 – } + {\text{16}}{{\text{H}}^ + } \to {\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{O}}_2}{\text{H}} + {\text{4C}}{{\text{r}}^{3 + }} + {\text{11}}{{\text{H}}_2}{\text{O}}\)
correct formulas of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} – }{\text{/}}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\) as reactants and
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H/C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and \({\text{C}}{{\text{r}}^{3 + }}\) as products;
full balanced chemical equation;
M2 can only be scored if M1 is correct.
Allow full balanced chemical equation to produce ethanal,
\({\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – } + {\text{8}}{{\text{H}}^ + } \to {\text{3C}}{{\text{H}}_3}{\text{CHO}} + {\text{2C}}{{\text{r}}^{3 + }} + {\text{7}}{{\text{H}}_2}{\text{O}}\).
Accept full or condensed structural formulas.
ethanoic acid;
Allow acetic acid.
Allow ethanal/acetaldehyde.
infrared (spectroscopy)/IR;
CH characteristic band (at \({\text{2950 c}}{{\text{m}}^{ – 1}}\)) for ethanol / C–H bonds in ethanol absorb at certain frequency/wavelength;
Do not award M2 for CH characteristic band if however wavenumber range/value is given for OH (eg, 3200–3600 cm–1 or value in between or even 2500–3300 cm–1).
area under peak used to measure concentration (of ethanol);
Accept “size of” instead of “area under”.
Do not accept “height” instead of “area under”.
OR
fuel cell;
ethanol converts/oxidized to \({\text{C}}{{\text{O}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\);
(energy released converted to) voltage/potential difference (which is) proportional to/can be used to measure concentration (of ethanol);
Allow potential instead of potential difference.
Examiners report
Only about half the candidates gave the correct formula for potassium dichromate(VI).
Most candidates knew the colour change.
The oxidation number was often given using incorrect notation (3+ or 3) failing to score the mark.
The redox equation was challenge except for the strongest candidates.
About half the candidates gave the correct product for the oxidation of ethanol.
Part (c) was poorly answered. About half of the candidates scored one mark for recognizing that the intoximeter used IR radiation. Few candidates gained a second mark for recognizing that the absorption by C-H bonds is used to determine ethanol concentration. It was rare to see an answer mentioning the area under the peak or using the fuel cell in the intoximeter.
Question
Dissolved oxygen is used up when organic matter is decomposed aerobically in water.
The Winkler method, which is based on redox reactions, can be used to determine the concentration of dissolved oxygen in water.
A \({\text{200 c}}{{\text{m}}^{\text{3}}}\) sample of water was taken from a river and analysed using this method.
The redox reactions are shown below.
Step 1 \({\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ – }{\text{(aq)}} + {{\text{O}}_2}{\text{(aq)}} \to {\text{2Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Step 2 \({\text{Mn}}{{\text{O}}_2}{\text{(s)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Step 3 \({\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 – }{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} \to {{\text{S}}_4}{\text{O}}_6^{2 – }{\text{(aq)}} + {\text{2}}{{\text{I}}^ – }{\text{(aq)}}\)
(i) \(1.50 \times {10^{ – 4}}{\text{ mol}}\) of \({{\text{I}}^ – }{\text{(aq)}}\) was formed in step 3. Determine the amount, in mol, of oxygen, \({{\text{O}}_{\text{2}}}{\text{(aq)}}\), dissolved in the water.
(ii) Determine the solubility, in \({\text{g}}\,{\text{d}}{{\text{m}}^{ – 3}}\), of the oxygen in the water.
▶️Answer/Explanation
Markscheme
(i) \(\left( {\frac{{1.50 \times {{10}^{ – 4}}}}{4} = } \right){\text{ }}3.75 \times {10^{ – 5}}{\text{ (mol)}}\);
(ii) \(\left( {\frac{{3.75 \times {{10}^{ – 5}} \times 1000 \times 32.00}}{{200}} = } \right){\text{ }}6.00 \times {10^{ – 3}}{\text{ }}({\text{g}}\,{\text{d}}{{\text{m}}^{ – 3}})\);
Examiners report
Candidates performed poorly on this question and were not able to gain the mark for part (a). Many did not apply the terms oxidation and reduction correctly and some did not distinguish between the two terms, only one of the two terms was explained. Some incorrectly stated hydrogen used in anaerobic respiration instead of oxygen being absent. Several also missed reference to organic material. Several candidates were able to perform the calculations, for part (b), correctly; some used incorrect value for \({{\text{I}}^ – }\) moles (2 instead of 4) in part (ii) and did not gain the mark.
Question
In order to provide safe drinking water, a water supply is often treated with disinfectants, which aim to inactivate disease-causing bacteria in the water.
To compare the effectiveness of different disinfectants, a CT value is used as a measure of the dosage of disinfectant needed to achieve a certain level of inactivation of specific bacteria.
CT value (mg min dm−3) = C (mg dm−3) concentration of disinfectant × T (min) contact time with water
The table below compares the CT values of different disinfectants necessary to achieve 99% inactivation of two types of bacteria, listed as A and B.
(i) Deduce the oxidation state of chlorine in the following disinfectants.
(ii) From the data on CT values, justify the statement that bacterium B is generally more resistant to disinfection than bacterium A.
(iii) CT values can be used to determine whether a particular treatment process is adequate. Calculate the CT value, in mg min dm−3, when 1.50 × 10−5 g dm−3 of chlorine dioxide is added to a water supply with a contact time of 9.82 minutes.
(iv) From your answer to (a) (iii) and the data in the table, comment on whether this treatment will be sufficient to inactivate 99% of bacterium A.
CT values are influenced by temperature and by pH. The table below shows the CT values for chlorine needed to achieve 99% inactivation of a specific bacterium at stated values of pH and temperature.
(i) With reference to the temperature data in the table, suggest why it may be more difficult to treat water effectively with chlorine in cold climates.
(ii) Sketch a graph on the axes below to show how the CT value (at any temperature) varies with pH.
(iii) Comment on the relative CT values at pH 6.0 and pH 9.0 at each temperature.
(iv) Chlorine reacts with water as follows:
Cl2 (g) + H2O (l) \( \rightleftharpoons \) HOCl (aq) + HCl (aq)
HOCl (aq) \( \rightleftharpoons \) OCl− (aq) + H+ (aq)
Predict how the concentrations of each of the species HOCl (aq) and OCl− (aq) will change if the pH of the disinfected water increases.
Despite widespread improvements in the provision of safe drinking water, the sale of bottled water has increased dramatically in recent years. State one problem caused by this trend.
▶️Answer/Explanation
Markscheme
i
HOCl: +1
AND
ClO2: +4
Accept “I” and “IV” but not “1+/1” and “4+/4” notations.
ii
«most» CT values are higher for «bacterium» B
OR
«generally» higher dosage needed for «bacterium» B
Accept converse arguments. Accept “concentration” for “dosage”
iii
«CT = 1.50 × 10–5 × 103 mg dm–3 × 9.82 min =» 1.47 × 10–1 «mg min dm–3»
iv
lower than CT value/minimum dosage/1.8 × 10–1 «mg min dm–3»
AND
no/insufficient
Accept “concentration” for “dosage”.
i
higher CT value at lower temperature
OR
higher dosage «of chlorine» needed at low temperature
Accept “effectiveness decreases at lower temperature”.
Accept “concentration” for “dosage”.
Accept converse arguments.
ii
labeled axes ( y: CT and x: pH)
AND
curve with increasing gradient
Do not accept axes the wrong way round.
Accept a linear sketch.
iii
values at pH 9.0 approximately 3 times values at pH 6.0
OR
increase in CT values in same ratio
The exact ratio is 2.9 times
Do not accept just “increase in value”.
iv
[HOCl] decreases AND [OCl−] increases
plastic disposal/pollution
OR
plastic bottles use up petroleum/non-renewable raw material
OR
chemicals in plastic bottle can contaminate water
OR
«prolonged» storage in plastic can cause contamination of water
OR
plastic water bottles sometimes reused without proper hygiene considerations
Accept other valid answers.
Accept economic considerations such as “greater production costs”, “greater transport costs” or “bottled water more expensive than tap water”
Question
Rhodium and palladium are often used together in catalytic converters. Rhodium is a good reduction catalyst whereas palladium is a good oxidation catalyst.
In a catalytic converter, carbon monoxide is converted to carbon dioxide. Outline the process for this conversion referring to the metal used.
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
Deduce the redox equation for the reaction of nickel(II) chloride solution with the metal identified in (b)(i).
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
▶️Answer/Explanation
Markscheme
carbon monoxide/CO adsorbs onto palladium/Pd
bonds stretched/weakened/broken
OR
«new» bonds formed
OR
activation energy/Ea «barrier» lowered «in both forward and reverse reactions»
products/CO2 desorb «from catalyst surface»
[3 marks]
Fe/iron
OR
Zn/zinc
OR
Co/cobalt
OR
Cd/cadmium
OR
Cr/chromium
Accept “Mn/manganese”.
[1 mark]
Ni2+(aq) + Fe(s) → Ni(s) + Fe2+(aq)
OR
Ni2+(aq) + Zn(s) → Ni(s) + Zn2+(aq)
OR
Ni2+(aq) + Co(s) → Ni(s) + Co2+(aq)
OR
Ni2+(aq) + Cd(s) → Ni(s) + Cd2+(aq)
OR
Ni2+(aq) + Cr(s) → Ni(s) + Cr2+(aq)
Accept “3Ni2+(aq) + 2Cr(s) → 3Ni(s) + 2Cr3+(aq)”.
Do not penalize similar equations involving formation of Fe3+(aq), Mn2+(aq) OR Co3+(aq).
Ignore Cl− ions.
Accept correctly balanced non-ionic equations eg, “NiCl2(aq) + Zn(s) → Ni(s) + ZnCl2(aq)” etc.
Do not allow ECF from (b)(i).
[2 mark]
\(n{\text{(}}{{\text{e}}^ – }{\text{)}}\) «\( = \frac{{2.50{\text{ A}} \times 3600{\text{ s}}}}{{96500{\text{ C}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}\)» = 0.09326 «mol»
OR
\(n{\text{(Ni)}}\) «\( = \frac{{0.09326{\text{ mol}}}}{2}\)» = 0.04663 «mol»
\(m{\text{(Ni)}}\) «= 0.04663 mol x 58.69 g\(\,\)mol–1» = 2.74 «g»
Award [2] for correct final answer.
[2 marks]

