Question
(i) The diagram shows an unlabelled voltaic cell for the reaction
Pb2+ (aq) + Ni (s) → Ni2+ (aq) + Pb (s)
Label the diagram with the species in the equation. [1]
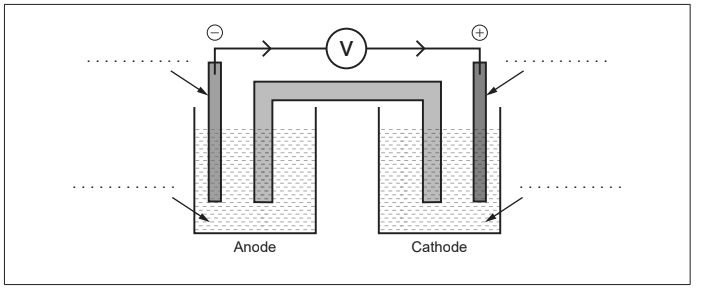
(ii) Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet. [1]
(iii) Describe the bonding in metals. [2]
(iv) Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel. [1]
▶️Answer/Explanation
Ans
d i 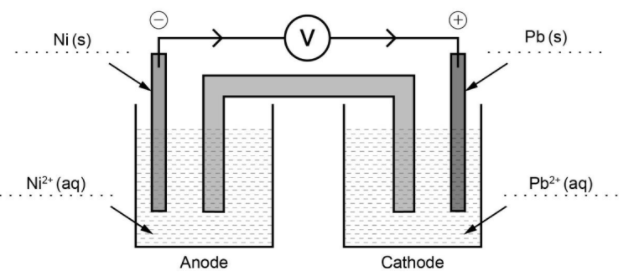
d ii Bi/Cu/Ag/Pd/Hg/Pt/Au Accept Sb OR As.
d iii electrostatic attraction between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
d iv
Any of:
malleability/hardness
OR
«tensile» strength/ductility
OR
density
OR
thermal/electrical conductivity
OR
melting point
OR
thermal expansion ✔
Question
Oxidation and reduction reactions can have a variety of commercial uses.
(a) A student decides to build a voltaic cell consisting of an aluminium electrode, Al (s), a tin electrode, Sn (s), and solutions of aluminium nitrate, Al(NO3)3 (aq) and tin(II) nitrate, Sn(NO3)2 (aq).
Electron flow is represented on the diagram.
Label each line in the diagram using data booklet. [3]
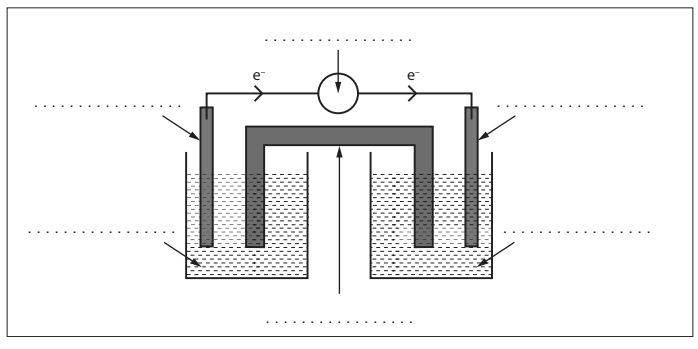
(b) Write the equation for the expected overall chemical reaction in (a). [1]
▶️Answer/Explanation
Ans:
a
Al/aluminium «electrode» AND aluminium nitrate/Al(NO3)3/Al 3+ on left
Sn/tin «electrode» AND tin«(II)» nitrate/Sn(NO3)2/Sn2+ on right
salt bridge AND voltmeter/V/lightbulb
Award [1] if M1 and M2 are reversed.
Award [1] for two correctly labelled solutions OR two correctly labelled electrodes for M1 and M2. Accept a specific salt for “salt bridge”.
Accept other circuit components such as ammeter/A, fan, buzzer, resistor/heating element/R/Ω.
b
3Sn2+ (aq) + 2Al (s) → 3Sn (s) + 2Al 3+ (aq)
OR 3Sn(NO3)2 (aq) + 2Al(s) → 3Sn (s) + 2Al(NO3)3 (aq)
If half-cells are reversed in (a) then the equation must be reversed to award the mark. Do not penalize equilibrium arrow
Question
Sodium oxide, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\), is a white solid with a high melting point.
a.Explain why solid sodium oxide is a non-conductor of electricity.[1]
b.Molten sodium oxide is a good conductor of electricity. State the half-equation for the reaction occurring at the positive electrode during the electrolysis of molten sodium oxide.[1]
c.i.State the acid-base nature of sodium oxide.[1]
c.ii.State the equation for the reaction of sodium oxide with water.[1]
▶️Answer/Explanation
Markscheme
in the solid state ions are in fixed positions/there are no moveable ions / OWTTE;
Do not accept answer that refers to atoms or molecules.
\({\text{2}}{{\text{O}}^{2 – }} \to {{\text{O}}_2} + {\text{4}}{{\text{e}}^ – }/{{\text{O}}^{2 – }} \to \frac{1}{2}{{\text{O}}_2} + {\text{2}}{{\text{e}}^ – }\);
Accept e instead of e–.
basic;
Allow alkaline
\({\text{N}}{{\text{a}}_2}{\text{O}} + {{\text{H}}_2}{\text{O}} \to {\text{2NaOH}}/{\text{N}}{{\text{a}}_2}{\text{O}} + {{\text{H}}_2}{\text{O}} \to {\text{2N}}{{\text{a}}^ + } + {\text{2O}}{{\text{H}}^ – }\);
Do not accept \( \rightleftharpoons \)
Examiners report
This was expected to be a high-scoring question but this was not found in practice. In Part (a) there were many references to delocalised/mobile electrons and also molecules and atoms. It did not appear that the structural properties of ionic substances are well understood.
There were many attempts in (b) which involved the sodium ion rather than the oxide and those who chose oxide often had difficulty in producing a balanced equation.
The best answered part of this question was Part (c) though a significant percentage described it as a weak base.
The best answered part of this question was Part (c) though a significant percentage described it as a weak base.
