Question
There has been significant growth in the use of carbon nanotubes, CNT.
a. Explain these properties of carbon nanotubes.
b(i)Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium-CNT alloy.
b(ii)Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
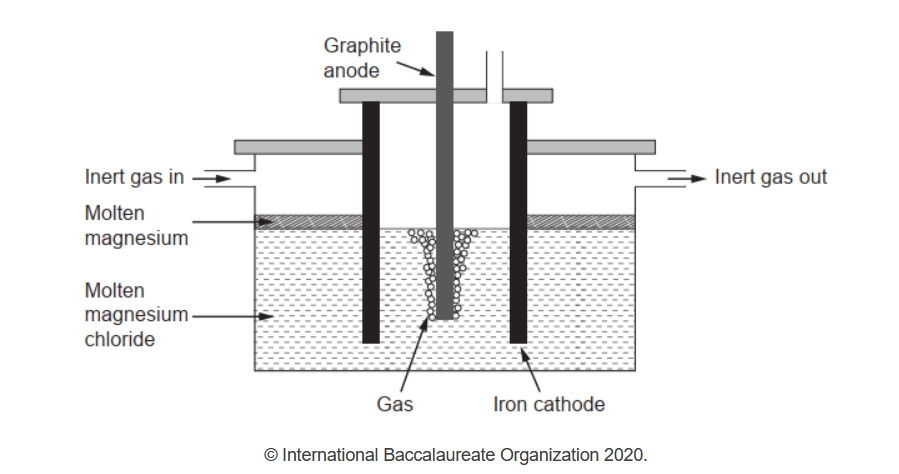
Write the half-equations for the reactions occurring in this electrolysis.
$\mathrm{b}$ (iiifalculate the theoretical mass of magnesium obtained if a current of $3.00 \mathrm{~A}$ is used for 10.0 hours. Use charge $(Q)=\operatorname{current}(I) \times \operatorname{time}(t)$ and section 2 of the data booklet
b(i\s,uggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
c. Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
d. Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
▶️Answer/Explanation
Markscheme
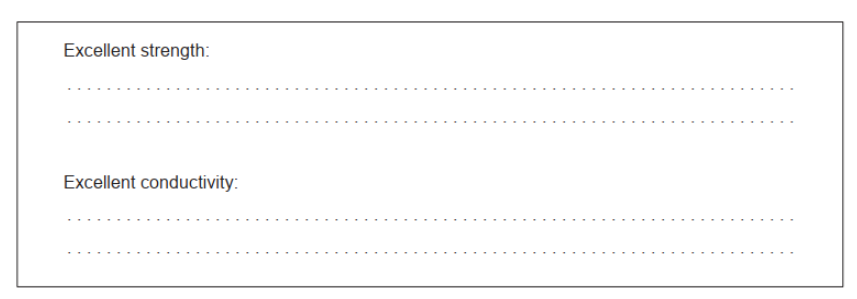
a. Excellent strength: defect-free AND rigid/regular 2D/3D $\sim$
Excellent conductivity: delocalized electrons
Accept “carbons/atoms are all covalently bonded to each other” for M1.
b(i)Any of:
ductility $\sim$
strength/resistance to deformation
malleability
hardness $\checkmark$
resistance to corrosion/chemical resistance
range of working temperatures $\checkmark$
density
Do not accept “conductivity”.
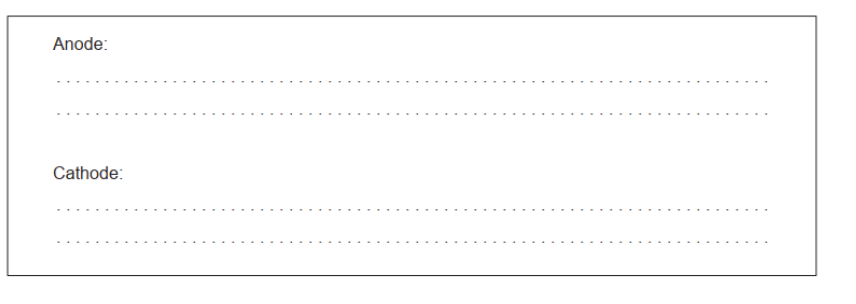
b(ii)Anode: $2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-}$
Cathode: $\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg}(1)$
Accept $\mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+e^{-}$.
Award [1 max] for correct equations at incorrect electrodes.
$$
\begin{aligned}
& \mathrm{b}(\mathrm{iii}) Q=I \times t=3.00 \times 10.0 \times 3600=» 108000 \mathrm{C} \\
& « \frac{Q}{F}=\frac{108000 \mathrm{C}}{96500 \mathrm{Cmol}^{-1}}=» 1.12 \ll \mathrm{mol} \mathrm{e}^{-} » \\
& \text { « } \frac{1.12 \mathrm{~mol}}{2}=0.560 \mathrm{~mol} \mathrm{Mg} » \\
& \left\langle m=0.560 \mathrm{~mol} \times 24.31 \mathrm{~g} \mathrm{~mol}^{-1}=» 13.6 \ll \mathrm{g}\right\rangle \\
&
\end{aligned}
$$
Award [3] for correct final answer.
b(ivęrgon/Ar/helium/He
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen $\mathrm{N}_2$ “.
c. pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react
Accept “molecules/ions” for “reactants” in M2.
d. rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order $\boldsymbol{A N D}$ have «some» directional order/pattern
Accept “linear” for “rod-shaped”.
Question
Aluminium is chemically reactive so it has to be extracted by the electrolysis of aluminium oxide dissolved in molten cryolite.
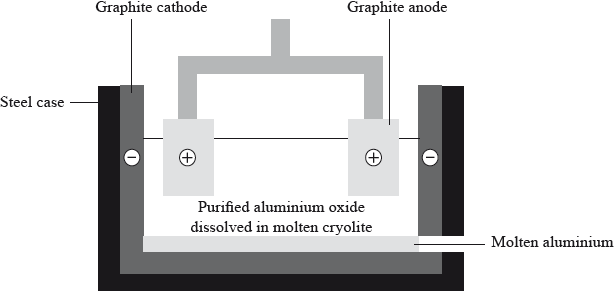
Deduce an equation for the discharge of the ions at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(i) Outline why aluminium is alloyed with copper and magnesium when used to construct aircraft bodies.
(ii) State two properties of aluminium that make it suitable for use in overhead power cables.
▶️Answer/Explanation
Markscheme
Positive electrode (anode):
\({\text{2}}{{\text{O}}^{2 – }} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{e}}^ – }/{{\text{O}}^{2 – }} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ – }/{\text{2}}{{\text{O}}^{2 – }} – {\text{4}}{{\text{e}}^ – } \to {{\text{O}}_2}{\text{(g)}}/\)
\({{\text{O}}^{2 – }} – {\text{2}}{{\text{e}}^ – } \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}}\);
Allow C(s) + 2O2– \( \to \) CO2(g) + 4e–.
Negative electrode (cathode):
\({\text{A}}{{\text{l}}^{3 + }} + {\text{3}}{{\text{e}}^ – } \to {\text{Al(l)}}\);
Accept e instead of e–.
Ignore state symbols.
If correct equations shown at wrong electrodes, award [1 max].
(i) harder/stronger (than pure aluminium);
(ii) Award [1] for any two of:
good conductor of electricity;
resists corrosion;
Do not allow rusting.
low density;
Do not allow lighter/light mass/light weight.
ductile;
Do not allow malleable.
Examiners report
In (a), most were able to write the correct half-equation for the cathode though incorrect states were commonly seen, e.g. (aq). The anode half-equation was not well known.
Both parts of (b) were well done. In (ii), incorrect answers included malleability and light mass.
Question
A student set up a simple voltaic cell consisting of a copper electrode and a zinc electrode dipped in sodium chloride solution.
The student gradually increased the distance, d, between the electrodes to study the effect on the initial current, I, passing through the light bulb.
The student hypothesized that the initial current would be inversely proportional to the distance between the electrodes.
The following data was collected over five trials.
The data did not support the student’s hypothesis. He investigated other possible relationships by plotting a graph of the average current against the distance between the electrodes. He obtained the following best-fit line with a correlation coefficient (r) of −0.9999.
Sketch a graph that would support the student’s hypothesis.
Suggest what the correlation coefficient of −0.9999 indicates.
State the equation of the straight line obtained using the data.
Outline how current flows in the sodium chloride solution.
▶️Answer/Explanation
Markscheme
OR
OR
Correct labels of axes required for mark.
Accept d–1 instead of \(\frac{1}{d}\).
Accept I–1 instead of \(\frac{1}{I}\).
Plot of I vs d should not be linear.
negative correlation
OR
model/prediction matches results
OR
99% of variance accounted for
I = – 0.001631 d + 0.09939
OR
y = – 0.001631 x + 0.09939
Accept correctly rounded values for m and b in equation.
Do not accept “y = mx + b”.
ions move «across electrolyte»

