Question
Which technique is used to determine the bond lengths and bond angles of a molecule?
A. X-ray crystallography
B. Infrared (IR) spectroscopy
C. Mass spectroscopy
D. 1H NMR spectroscopy
▶️Answer/Explanation
A
Single-crystal X-ray Diffraction is a non-destructive analytical technique which provides detailed information about the internal lattice of crystalline substances, including unit cell dimensions, bond-lengths, bond-angles, and details of site-ordering.
Question
Which feature of a molecule can be determined from its 1H NMR spectrum?
A. Number of hydrogen environments
B. Total mass of hydrogen atoms present
C. Vibration frequency of C–H bonds
D. Ionization energy of a hydrogen atom
▶️Answer/Explanation
A
If the 1H NMR spectrum is available for an unknown compound, counting the number of signals in the spectrum tells us the number of different sets of protons in the molecule, and that is the very important information to determine the structure of the compound. Therefore, we can determine the number of different hydrogen environments in the molecule.
Question
What is the index of hydrogen deficiency (IHD) in cyclohexanol?
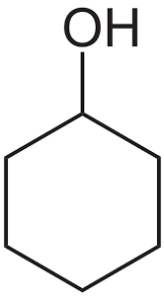
A 0
B 1
C 2
D 3
▶️Answer/Explanation
Ans: B
It has formula C6H12O.
If you have a molecular formula, CcHhNnOoXx, then the following equation: IHD = 0.5 * [2c+2-h-x+n]
Here, IHD = 0.5 * [2*6+2-12] =1.
Question
Which molecule has an index of hydrogen deficiency (IHD) = 1?
A. C6H6
B. C2Cl2
C. C4H9N
D. C2H6O
▶️Answer/Explanation
C
The Index of Hydrogen Deficiency (IHD), is a count of how many molecules of H2 need to be added to a structure in order to obtain the corresponding saturated, acyclic species.
If you have a molecular formula, CcHhNnOoXx, then the following equation: IHD = 0.5 * [2c+2-h-x+n]
For C6H6 , IHD = 0.5*[2*6+2-6] = 4.
For C2Cl2, Each Cl atom is treated as H for calculating IHD i.e. C2H2 IHD = 0.5*[2*2+2-2] = 2.
C4H9N, IHD = 0.5*[2*4+2-9+1] = 1.
C2H6O, IHD = 0.5*[2*2+2-6] = 0.
