Question
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.[2]
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.[1]
The structural formula of urea is shown.
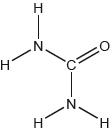
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
 [3]
[3]
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution[2]
State the equilibrium constant expression, Kc.[1]
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.[1]
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.[2]
Suggest one reason why urea is a solid and ammonia a gas at room temperature.[1]
Sketch two different hydrogen bonding interactions between ammonia and water.[2]
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.[2]
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.[1]
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.[2]
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.[1]
The mass spectrum of urea is shown below.
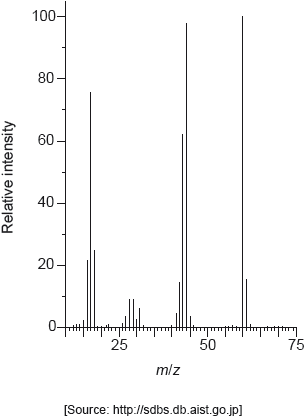
Identify the species responsible for the peaks at m/z = 60 and 44.
[2]
The IR spectrum of urea is shown below.
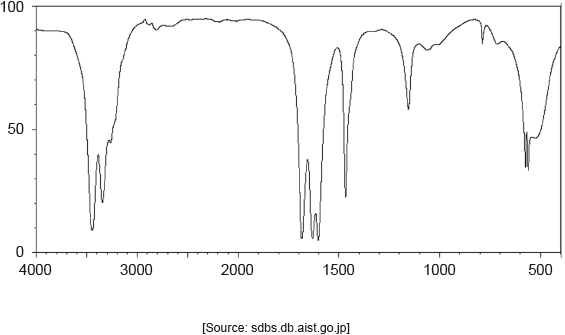
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
[2]
Predict the number of signals in the 1H NMR spectrum of urea.[1]
Predict the splitting pattern of the 1H NMR spectrum of urea.[1]
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.[2]
Answer/Explanation
Markscheme
molar mass of urea «4 \( \times \) 1.01 + 2 \( \times \) 14.01 + 12.01 + 16.00» = 60.07 «g mol-1»
«% nitrogen = \(\frac{{{\text{2}} \times {\text{14.01}}}}{{{\text{60.07}}}}\) \( \times \) 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]

Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 \( \times \) 0.100 mol dm–3» = 5.00 \( \times \) 10–3 «mol»
«mass of urea = 5.00 \( \times \) 10–3 mol \( \times \) 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
\({K_{\text{c}}} = \frac{{[{{({{\text{H}}_2}{\text{N}})}_2}{\text{CO}}] \times [{{\text{H}}_2}{\text{O}}]}}{{{{[{\text{N}}{{\text{H}}_3}]}^2} \times [{\text{C}}{{\text{O}}_2}]}}\)
[1 mark]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
ln K « = \(\frac{{ – \Delta {G^\Theta }}}{{RT}} = \frac{{ – 50 \times {{10}^3}{\text{ J}}}}{{8.31{\text{ J }}{{\text{K}}^{ – 1}}{\text{ mo}}{{\text{l}}^{ – 1}} \times 298{\text{ K}}}}\) » = –20
«Kc =» 2 \( \times \) 10–9
OR
1.69 \( \times \) 10–9
OR
10–9
Accept range of 20-20.2 for M1.
Award [2] for correct final answer.
[2 marks]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]
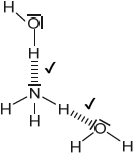
Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + \(\frac{3}{2}\)O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
«V = \(\frac{{{\text{0.600 g}}}}{{{\text{60.07 g mo}}{{\text{l}}^{ – 1}}}}\) \( \times \) 22700 cm3 mol–1 =» 227 «cm3»
[1 mark]
lone/non-bonding electron pairs «on nitrogen/oxygen/ligand» given to/shared with metal ion
co-ordinate/dative/covalent bonds
[2 marks]
lone pairs on nitrogen atoms can be donated to/shared with C–N bond
OR
C–N bond partial double bond character
OR
delocalization «of electrons occurs across molecule»
OR
slight positive charge on C due to C=O polarity reduces C–N bond length
[1 mark]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[2 marks]
singlet
Accept “no splitting”.
[1 mark]
acts as internal standard
OR
acts as reference point
one strong signal
OR
12 H atoms in same environment
OR
signal is well away from other absorptions
Accept “inert” or “readily removed” or “non-toxic” for M1.
[2 marks]
